Elements and Isotopes
 How are all of the isotopes of an element similar?
How are all of the isotopes of an element similar?
A chemical element is a pure substance that consists entirely of one type of atom. More than 100 elements are known, but only about two dozen are commonly found in living organisms. Elements are represented by one- or two-letter symbols. C, for example, stands for carbon, H for hydrogen, Na for sodium, and Hg for mercury. The number of protons in the nucleus of an element is called its atomic number. Carbon's atomic number is 6, meaning that each atom of carbon has six protons and, consequently, six electrons. See Appendix E, The Periodic Table, which shows the elements.
Isotopes Atoms of an element may have different numbers of neutrons. For example, although all atoms of carbon have six protons, some have six neutrons, some seven, and a few have eight. Atoms of the same element that differ in the number of neutrons they contain are known as isotopes. The total number of protons and neutrons in the nucleus of an atom is called its mass number. Isotopes are identified by their mass numbers. Figure 2–3 shows the subatomic composition of carbon-12, carbon-13, and carbon-14 atoms. The weighted average of the masses of an element's isotopes is called its atomic mass. “Weighted” means that the abundance of each isotope in nature is considered when the average is calculated.  Because they have the same number of electrons, all isotopes of an element have the same chemical properties.
Because they have the same number of electrons, all isotopes of an element have the same chemical properties.

FIGURE 2–2 Droplets of Mercury Mercury, a silvery-white metallic element, is liquid at room temperature and forms droplets. It is extremely poisonous.
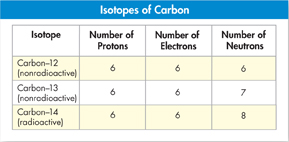
FIGURE 2–3 Carbon Isotopes Isotopes of carbon all have 6 protons but different numbers of neutrons—6, 7, or 8. They are identified by the total number of protons and neutrons in the nucleus: carbon–12, carbon–13, and carbon–14. Classify Which isotope of carbon is radioactive?
ddddRadioactive Isotopes Some isotopes are radioactive, meaning that their nuclei are unstable and break down at a constant rate over time. The radiation these isotopes give off can be dangerous, but radioactive isotopes have a number of important scientific and practical uses.
Geologists can determine the ages of rocks and fossils by analyzing the isotopes found in them. Radiation from certain isotopes can be used to detect and treat cancer and to kill bacteria that cause food to spoil. Radioactive isotopes can also be used as labels or “tracers” to follow the movements of substances within organisms.
 In Your Notebook Draw a diagram of a helium atom, which has an atomic number of 2.
In Your Notebook Draw a diagram of a helium atom, which has an atomic number of 2.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis