2.2 Properties of Water
 How does the structure of water contribute to its unique properties?
How does the structure of water contribute to its unique properties? How does water's polarity influence its properties as a solvent?
How does water's polarity influence its properties as a solvent? Why is it important for cells to buffer solutions against rapid changes in pH?
Why is it important for cells to buffer solutions against rapid changes in pH?
hydrogen bond • cohesion • adhesion • mixture • solution • solute • solvent • suspension • pH scale • acid • base • buffer
Venn Diagram As you read, draw a Venn diagram showing the differences between solutions and suspensions and the properties that they share.
THINK ABOUT IT Looking back at our beautiful planet, an astronaut in space said that if other beings have seen the Earth, they must surely call it “the blue planet.” He referred, of course, to the oceans of water that cover nearly three fourths of Earth's surface. The very presence of liquid water tells a scientist that life may also be present on such a planet. Why should this be so? Why should life itself be connected so strongly to something so ordinary that we often take it for granted? The answers to those questions suggest that there is something very special about water and the role it plays in living things.
The Water Molecule
 How does the structure of water contribute to its unique properties?
How does the structure of water contribute to its unique properties?
Water is one of the few compounds found in a liquid state over most of the Earth's surface. Like other molecules, water (H2O) is neutral. The positive charges on its 10 protons balance out the negative charges on its 10 electrons. However, there is more to the story.
Polarity With 8 protons, water's oxygen nucleus attracts electrons more strongly than the single protons of water's two hydrogen nuclei. As a result, water's shared electrons are more likely to be found near the oxygen nucleus. Because the oxygen nucleus is at one end of the molecule, as shown in Figure 2–6, water has a partial negative charge on one end, and a partial positive charge on the other.
A molecule in which the charges are unevenly distributed is said to be “polar,” because the molecule is a bit like a magnet with two poles. The partial charges on a polar molecule are written in parentheses, (–) or (+), to show that they are weaker than the charges on ions such as Na+ and Cl–.
Hydrogen Bonding Because of their partial positive and negative charges, polar molecules such as water can attract each other. The attraction between a hydrogen atom with a partial positive charge and another atom with a partial negative charge is known as a hydrogen bond. The most common partially negative atoms involved in hydrogen bonding are oxygen, nitrogen, and fluorine.
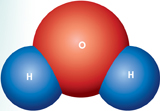
FIGURE 2–6 A Water Molecule A water molecule is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. The negative pole is near the oxygen atom and the positive pole is between the hydrogen atoms.

Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis