2.1 The Nature of Matter
 What three subatomic particles make up atoms?
What three subatomic particles make up atoms? How are all of the isotopes of an element similar?
How are all of the isotopes of an element similar? In what ways do compounds differ from their component elements?
In what ways do compounds differ from their component elements? What are the main types of chemical bonds?
What are the main types of chemical bonds?
atom • nucleus • electron • element • isotope • compound • ionic bond • ion • covalent bond • molecule • van der Waals forces
Outline Before you read, make an outline of the major headings in the lesson. As you read, fill in main ideas and supporting details under each head.
THINK ABOUT IT What are you made of ? Just as buildings are made from bricks, steel, glass, and wood, living things are made from chemical compounds. But it doesn't stop there. When you breathe, eat, or drink, your body uses the substances in air, food, and water to carry out chemical reactions that keep you alive. If the first task of an architect is to understand building materials, then what would be the first job of a biologist? Clearly, it is to understand the chemistry of life.
Atoms
 What three subatomic particles make up atoms?
What three subatomic particles make up atoms?
The study of chemistry begins with the basic unit of matter, the atom. The concept of the atom came first from the Greek philosopher Democritus, nearly 2500 years ago. Democritus asked a simple question: If you take an object like a stick of chalk and break it in half, are both halves still chalk? The answer, of course, is yes. But what happens if you break it in half again and again and again? Can you continue to divide without limit, or does there come a point at which you cannot divide the fragment of chalk without changing it into something else? Democritus thought that there had to be a limit. He called the smallest fragment the atom, from the Greek word atomos, which means “unable to be cut.”
Atoms are incredibly small. Placed side by side, 100 million atoms would make a row only about 1 centimeter long—about the width of your little finger! Despite its extremely small size, an atom contains subatomic particles that are even smaller. Figure 2–1 shows the subatomic particles in a carbon atom.  The subatomic particles that make up atoms are protons, neutrons, and electrons.
The subatomic particles that make up atoms are protons, neutrons, and electrons.
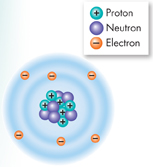
FIGURE 2–1 A Carbon Atom
Protons and Neutrons Protons and neutrons have about the same mass. However, protons are positively charged particles (+) and neutrons carry no charge at all. Strong forces bind protons and neutrons together to form the nucleus, at the center of the atom.
Electrons The electron is a negatively charged particle (–) with only 1/1840 the mass of a proton. Electrons are in constant motion in the space surrounding the nucleus. They are attracted to the positively charged nucleus but remain outside the nucleus because of the energy of their motion. Because atoms have equal numbers of electrons and protons, their positive and negative charges balance out, and atoms themselves are electrically neutral.

Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis