2.3 Carbon Compounds
 What elements does carbon bond with to make up life's molecules?
What elements does carbon bond with to make up life's molecules? What are the functions of each of the four groups of macromolecules?
What are the functions of each of the four groups of macromolecules?
monomer • polymer • carbohydrate • monosaccharide • lipid • nucleic acid • nucleotide • protein • amino acid
Compare/Contrast Table As you read, make a table that compares and contrasts the four groups of organic compounds.
THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. Today we understand that the principles governing the chemistry of living and nonliving things are the same, but the term “organic chemistry” is still around. Today, organic chemistry means the study of compounds that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds.
The Chemistry of Carbon
 What elements does carbon bond with to make up life's molecules?
What elements does carbon bond with to make up life's molecules?
Why is carbon so interesting that a whole branch of chemistry should be set aside just to study carbon compounds? There are two reasons for this. First, carbon atoms have four valence electrons, allowing them to form strong covalent bonds with many other elements.  Carbon can bond with many elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen to form the molecules of life. Living organisms are made up of molecules that consist of carbon and these other elements.
Carbon can bond with many elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen to form the molecules of life. Living organisms are made up of molecules that consist of carbon and these other elements.
Even more important, one carbon atom can bond to another, which gives carbon the ability to form chains that are almost unlimited in length. These carbon-carbon bonds can be single, double, or triple covalent bonds. Chains of carbon atoms can even close up on themselves to form rings, as shown in Figure 2–12. Carbon has the ability to form millions of different large and complex structures. No other element even comes close to matching carbon's versatility.
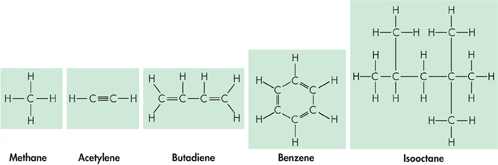
FIGURE 2–12 Carbon Structures Carbon can form single, double, or triple bonds with other carbon atoms. Each line between atoms in a molecular drawing represents one covalent bond. Observing How many covalent bonds are there between the two carbon atoms in acetylene?
d
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis