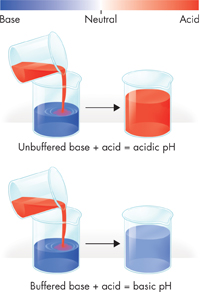
FIGURE 2–11 Buffers Buffers help prevent drastic changes in pH. Adding acid to an unbuffered solution causes the pH of the unbuffered solution to drop. If the solution contains a buffer, however, adding the acid will cause only a slight change in pH.
Acids Where do all those extra H+ ions in a low-pH solution come from? They come from acids. An acid is any compound that forms H+ ions in solution. Acidic solutions contain higher concentrations of H+ ions than pure water and have pH values below 7. Strong acids tend to have pH values that range from 1 to 3. The hydrochloric acid (HCl) produced by the stomach to help digest food is a strong acid.
Bases A base is a compound that produces hydroxide (OH–) ions in solution. Basic, or alkaline, solutions contain lower concentrations of H+ ions than pure water and have pH values above 7. Strong bases, such as the lye (commonly NaOH) used in soapmaking, tend to have pH values ranging from 11 to 14.
Buffers The pH of the fluids within most cells in the human body must generally be kept between 6.5 and 7.5. If the pH is lower or higher, it will affect the chemical reactions that take place within the cells. Thus, controlling pH is important for maintaining homeostasis. One of the ways that organisms control pH is through dissolved compounds called buffers. Buffers are weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in pH. Blood, for example, has a normal pH of 7.4. Sudden changes in blood pH are usually prevented by a number of chemical buffers, such as bicarbonate and phosphate ions.  Buffers dissolved in life's fluids play an important role in maintaining homeostasis in organisms.
Buffers dissolved in life's fluids play an important role in maintaining homeostasis in organisms.
2.2 Assessment

-
Review What does it mean when a molecule is said to be “polar”?
Explain How do hydrogen bonds between water molecules occur?
Use Models Use the structure of a water molecule to explain why it is polar.
-
Review Why is water such a good solvent?
Compare and Contrast What is the difference between a solution and a suspension?
-
Review What is an acid? What is a base?
Explain The acid hydrogen fluoride (HF) can be dissolved in pure water. Will the pH of the solution be greater or less than 7?
Infer During exercise, many chemical changes occur in the body, including a drop in blood pH, which can be very serious. How is the body able to cope with such changes?
WRITE ABOUT SCIENCE
Suppose you are a writer for a natural history magazine for children. This month's issue will feature insects. Write a paragraph explaining why some bugs, such as the water strider, can walk on water.

Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis