Acids, Bases, and pH
 Why is it important for cells to buffer solutions against rapid changes in pH?
Why is it important for cells to buffer solutions against rapid changes in pH?
Water molecules sometimes split apart to form ions. This reaction can be summarized by a chemical equation in which double arrows are used to show that the reaction can occur in either direction.
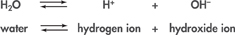
How often does this happen? In pure water, about 1 water molecule in 550 million splits to form ions in this way. Because the number of positive hydrogen ions produced is equal to the number of negative hydroxide ions produced, pure water is neutral.
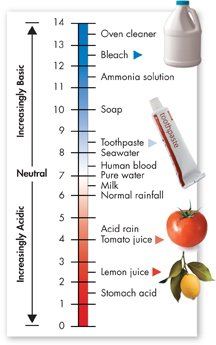
FIGURE 2–10 The pH Scale The concentration of H+ ions determines whether solutions are acidic or basic. The most acidic material on this pH scale is stomach acid. The most basic material on this scale is oven cleaner.
dThe pH Scale Chemists devised a measurement system called the pH scale to indicate the concentration of H+ ions in solution. As Figure 2–10 shows, the pH scale ranges from 0 to 14. At a pH of 7, the concentration of H+ ions and OH– ions is equal. Pure water has a pH of 7. Solutions with a pH below 7 are called acidic because they have more H+ ions than OH– ions. The lower the pH, the greater the acidity. Solutions with a pH above 7 are called basic because they have more OH– ions than H+ ions. The higher the pH, the more basic the solution. Each step on the pH scale represents a factor of 10. For example, a liter of a solution with a pH of 4 has 10 times as many H+ ions as a liter of a solution with a pH of 5.
 In Your Notebook Order these items in order of increasing acidity: soap, lemon juice, milk, acid rain.
In Your Notebook Order these items in order of increasing acidity: soap, lemon juice, milk, acid rain.
Quick Lab
GUIDED INQUIRY
Acidic and Basic Foods

Predict whether the food samples provided are acidic or basic.
Tear off a 2-inch piece of pH paper for each sample you will test. Place these pieces on a paper towel.
Construct a data table in which you will record the name and pH of each food sample.
Use a scalpel to cut a piece off each solid.
CAUTION: Be careful not to cut yourself. Do not eat the food. Touch the cut surface of each sample to a square of pH paper. Use a dropper pipette to place a drop of any liquid sample on a square of pH paper. Record the pH of each sample in your data table.
Analyze Data Were most of the samples acidic or basic?
Evaluate Was your prediction correct?
Analyze and Conclude
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis