Solutions and Suspensions
 How does water's polarity influence its properties as a solvent?
How does water's polarity influence its properties as a solvent?
Water is not always pure; it is often found as part of a mixture. A mixture is a material composed of two or more elements or compounds that are physically mixed together but not chemically combined. Salt and pepper stirred together constitute a mixture. So do sugar and sand. Earth's atmosphere is a mixture of nitrogen, oxygen, carbon dioxide, and other gases. Living things are in part composed of mixtures involving water. Two types of mixtures that can be made with water are solutions and suspensions.
MYSTERY CLUE 
The solubility of gases increases as temperatures decrease. Think about when a can of warm soda is opened—the carbon dioxide dissolved in it fizzes out more rapidly because the gas is less soluble at warm temperatures. How might the temperature of antarctic waters affect the amount of dissolved oxygen available for ice fish?
Solutions If a crystal of table salt is placed in a glass of warm water, sodium and chloride ions on the surface of the crystal are attracted to the polar water molecules. Ions break away from the crystal and are surrounded by water molecules, as illustrated in Figure 2–9. The ions gradually become dispersed in the water, forming a type of mixture called a solution. All the components of a solution are evenly distributed throughout the solution. In a saltwater solution, table salt is the solute—the substance that is dissolved. Water is the solvent—the substance in which the solute dissolves.  Water's polarity gives it the ability to dissolve both ionic compounds and other polar molecules.
Water's polarity gives it the ability to dissolve both ionic compounds and other polar molecules.
Water easily dissolves salts, sugars, minerals, gases, and even other solvents such as alcohol. Without exaggeration, water is the greatest solvent on Earth. But even water has limits. When a given amount of water has dissolved all of the solute it can, the solution is said to be saturated.
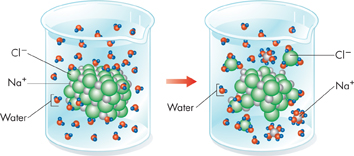
FIGURE 2–9 A Salt Solution When an ionic compound such as sodium chloride is placed in water, water molecules surround and separate the positive and negative ions.
Interpret Visuals What happens to the sodium ions and chloride ions in the solution?
Suspensions Some materials do not dissolve when placed in water, but separate into pieces so small that they do not settle out. The movement of water molecules keeps the small particles suspended. Such mixtures of water and nondissolved material are known as suspensions. Some of the most important biological fluids are both solutions and suspensions. The blood that circulates through your body is mostly water. The water in the blood contains many dissolved compounds. However, blood also contains cells and other undissolved particles that remain in suspension as the blood moves through the body.

Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis