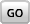Hydrogen bonds are not as strong as covalent or ionic bonds, but they give one of life's most important molecules many of its unique characteristics.  Because water is a polar molecule, it is able to form multiple hydrogen bonds, which account for many of water's special properties. These include the fact that water expands slightly upon freezing, making ice less dense than liquid water. Hydrogen bonding also explains water's ability to dissolve so many other substances, a property essential in living cells.
Because water is a polar molecule, it is able to form multiple hydrogen bonds, which account for many of water's special properties. These include the fact that water expands slightly upon freezing, making ice less dense than liquid water. Hydrogen bonding also explains water's ability to dissolve so many other substances, a property essential in living cells.
▸ Cohesion Cohesion is an attraction between molecules of the same substance. Because a single water molecule may be involved in as many as four hydrogen bonds at the same time, water is extremely cohesive. Cohesion causes water molecules to be drawn together, which is why drops of water form beads on a smooth surface. Cohesion also produces surface tension, explaining why some insects and spiders can walk on a pond's surface, as shown in Figure 2–7.
▸ Adhesion On the other hand, adhesion is an attraction between molecules of different substances. Have you ever been told to read the volume in a graduated cylinder at eye level? As shown in Figure 2–8, the surface of the water in the graduated cylinder dips slightly in the center because the adhesion between water molecules and glass molecules is stronger than the cohesion between water molecules. Adhesion between water and glass also causes water to rise in a narrow tube against the force of gravity. This effect is called capillary action. Capillary action is one of the forces that draws water out of the roots of a plant and up into its stems and leaves. Cohesion holds the column of water together as it rises.
▸ Heat Capacity Another result of the multiple hydrogen bonds between water molecules is that it takes a large amount of heat energy to cause those molecules to move faster, which raises the temperature of the water. Therefore, water's heat capacity, the amount of heat energy required to increase its temperature, is relatively high. This allows large bodies of water, such as oceans and lakes, to absorb large amounts of heat with only small changes in temperature. The organisms living within are thus protected from drastic changes in temperature. At the cellular level, water absorbs the heat produced by cell processes, regulating the temperature of the cell.
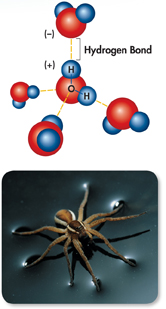
FIGURE 2–7 Hydrogen Bonding and Cohesion Each molecule of water can form multiple hydrogen bonds with other water molecules. The strong attraction between water molecules produces a force sometimes called “surface tension,” which can support very lightweight objects, such as this raft spider.
Apply Concepts Why are water molecules attracted to one another?
 In Your Notebook Draw a diagram of a meniscus. Label where cohesion and adhesion occur.
In Your Notebook Draw a diagram of a meniscus. Label where cohesion and adhesion occur.

FIGURE 2–8 Adhesion Adhesion between water and glass molecules is responsible for causing the water in these columns to rise. The surface of the water in the glass column dips slightly in the center, forming a curve called a meniscus.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis