2 Assessment
2.1 The Nature of Matter
Understand Key Concepts
The positively charged particle in an atom is called the
neutron.
ion.
proton.
electron.
Two or more different atoms are combined in definite proportions in any
symbol.
isotope.
element.
compound.
A covalent bond is formed by the
transfer of electrons.
sharing of electrons.
gaining of electrons.
losing of electrons.
Explain the relationship among atoms, elements, and compounds.
What is a radioactive isotope? Describe two scientific uses of radioactive isotopes.
Describe how the atoms in a compound are held together.
Distinguish among single, double, and triple covalent bonds.
Think Critically
Use Models Make a diagram like the one in Figure 2–4 to show how chlorine and hydrogen form from the compound hydrogen chloride, HCl.
Calculate A nanometer (nm) is one billionth of a meter (1 nm = 10−9 m). If 100 million atoms make a row 1 cm in length, what is the diameter of one atom in nanometers?

2.2 Properties of Water
Understand Key Concepts
When you shake sugar and sand together in a test tube, you cause them to form a
compound.
mixture.
solution.
suspension.
A compound that produces hydrogen ions in solution is a(n)
salt.
acid.
base.
polymer.
Compared to most other substances, a great deal of heat is needed to raise the temperature of water by a given amount. This is because water
is an acid.
readily forms solutions.
has a high heat capacity.
acts as a buffer.
Explain the properties of cohesion and adhesion. Give an example of each property.
What is the relationship among solutions, solutes, and solvents?
How are acids and bases different? How do their pH values differ?
Think Critically
Propose a Solution Silica is a hard, glassy material that does not dissolve in water. Suppose sodium chloride is accidentally mixed with silica. Describe a way to remove the sodium chloride.
Predict As part of the digestive process, the human stomach produces hydrochloric acid, HCl. Sometimes excess acid causes discomfort. In such a case, a person might take an antacid such as magnesium hydroxide, Mg(OH)2. Explain how this substance can reduce the amount of acid in the stomach.
2.3 Carbon Compounds
Understand Key Concepts
What does the following formula represent?
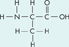
a sugar
a starch
an amino acid
a fatty acid
Proteins are polymers formed from
lipids.
carbohydrates.
amino acids.
nucleic acids.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis