Using Measurements in Calculations
Density is an example of a value that is calculated using two measurements. Density represents the amount of mass in a particular volume of a substance. The units used for density are grams per milliliter (g/mL) or grams per cubic centimeter (g/cm3). Density is calculated by dividing an object's mass by its volume.
Example
Follow these steps to calculate the density of an object.
Measure and record the mass of an object in grams.
Measure and record the volume of an object in mL or cm3.
Use the following formula to calculate density:
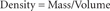
Effects of Measurement Errors
Density is calculated using two measured values. An error in the measurement of either mass or volume will result in the calculation of an incorrect density.
Example
A student measured the mass of an object as 2.5 g and its volume as 2.0 cm3. The actual mass of the object is 3.5 g; the actual volume is 2.0 cm3. What is the effect of the measurement error on the calculation of density?
Follow these steps to determine the effect of a measurement error on calculation.
Determine the density using the student's measurements.
Density = Mass/Volume
Density = 2.5 g/2.0 cm3
Density = 1.25 g/cm3
Determine the density using the actual values.
Density = Mass/Volume
Density = 3.5 g/2.0 cm3
Density = 1.75 g/cm3
Compare the calculated and the actual values.
In this case, a measurement of mass that was less than the actual value resulted in a calculated value for the density that was less than the actual density.
Accuracy
The accuracy of a measurement is its closeness to the actual value. Measurements that are accurate are close to the actual value.
Both clocks on this page show a time of 3:00. Suppose, though, that these clocks had not been changed to reflect daylight savings time. The time shown on the clocks would be inaccurate. On the other hand, if the actual time is 3:00, these clocks would be accurate.
Precision
Precision describes the exactness of a measurement. The clocks shown on this page differ in precision. The analog clock measures time to the nearest minute. The digital clock measures time to the nearest second. Time is measured more precisely by the digital clock than by the analog clock.

Comparing Accuracy and Precision
There is a difference between accuracy and precision. Measurements can be accurate (close to the actual value) but not precise. Measurements can also be precise but not accurate. When making scientific measurements, both accuracy and precision are important. Accurate and precise measurements result from the careful use of high-quality measuring tools.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis