After another half-life, another half of the remaining radioactive atoms will have decayed.  Radiometric dating uses the proportion of radioactive to stable isotopes to calculate the age of a sample.
Radiometric dating uses the proportion of radioactive to stable isotopes to calculate the age of a sample.
Quick Lab
GUIDED INQUIRY
Modeling Half-Life
1 Construct a data table or spreadsheet with two columns and five rows. Label the columns Spill Number and Number of Squares Returned. Take a sheet of paper, and cut out 100 1-cm squares. Place an X on each square, and put all the squares in a cup.
2 Mix the squares in the cup, and spill them out.
3 Remove all the squares that have an X showing. Count the squares left, record the number, and return the remaining squares to the cup.
4 Repeat steps 2 and 3 until there are five or fewer squares left. Make a graph of your results with the number of spills on the x-axis and the number of squares remaining after each spill on the y-axis.
Analyze Data How many spills did you need to remove half the squares? To remove three fourths?
Calculate If each spill represents one year, what is the half-life of the squares?

Analyze and Conclude
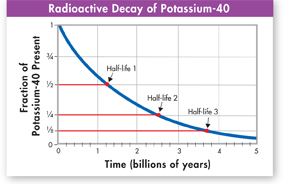
FIGURE 19–4 Radioactive Decay A half-life is the time it takes half the radioactive atoms in a sample to decay. The half-life of potassium-40 is 1.26 billion years.
dDifferent radioactive isotopes decay at different rates, so they have different half-lives. Elements with short half-lives are used to date recent fossils. Elements with long half-lives are used for dating older fossils. To understand this, think of timing sports events. For a 50-yard dash, a coach depends on the fast-moving second hand of a stopwatch. To time a marathon, slower-moving hour and minute hands are also important.
A number of radioactive isotopes are used to determine the ages of rocks and fossils. An isotope known as carbon-14 is particularly useful for directly dating organisms that lived in the recent past. Carbon-14 is produced at a steady rate in the upper atmosphere, so air generally contains a tiny amount of it, in addition to the much more common stable, nonradioactive form, carbon-12. Plants take carbon-14 in when they absorb carbon dioxide during photosynthesis, and animals acquire it when they eat plants or other animals. Once an organism dies, it no longer takes in this isotope, so its age can be determined by the amount of carbon-14 still remaining in tissues such as bone, hair, or wood. Carbon-14 has a half-life of roughly 5730 years, so its use is limited to organisms that lived in the last 60,000 years.
Older fossils can be dated indirectly by dating the rock layers in which they are found. Isotopes with much longer half-lives are used for this purpose, including potassium-40 (half-life: 1.26 billion years, shown in Figure 19–4), uranium-238 (4.5 billion years), and rubidium-87 (48.8 billion years). Over many years, geologists have combined the use of these and other isotope methods to make increasingly accurate estimates of the ages of geological formations. These studies have provided direct physical evidence for the ages of the index fossils used to identify periods of Earth history.
 In Your Notebook Explain why carbon-14 can't be used to estimate the age of very old fossils.
In Your Notebook Explain why carbon-14 can't be used to estimate the age of very old fossils.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis