Osmosis: An Example of Facilitated Diffusion Surprising new research has added water to the list of molecules that enter cells by facilitated diffusion. Recall that the inside of a cell's lipid bilayer is hydrophobic, or “water-hating.” Because of this, water molecules have a tough time passing through the cell membrane. However, many cells contain water channel proteins, known as aquaporins (ak wuh PAWR inz), that allow water to pass right through them, as shown in Figure 7–16. The movement of water through cell membranes by facilitated diffusion is an extremely important biological process—the process of osmosis.
Osmosis is the diffusion of water through a selectively permeable membrane. In osmosis, as in other forms of diffusion, molecules move from an area of higher concentration to an area of lower concentration. The only difference is that the molecules that move in the case of osmosis are water molecules, not solute molecules. The process of osmosis is shown in Figure 7–17.
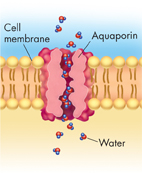
FIGURE 7–16 An Aquaporin
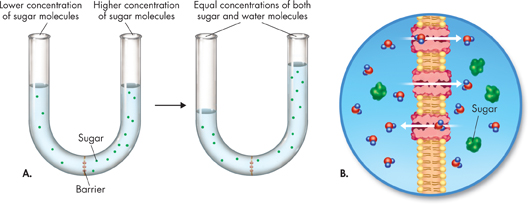
FIGURE 7–17 Osmosis Osmosis is a form of facilitated diffusion. A. In a laboratory experiment, water moves through a selectively permeable barrier from an area of lower to higher solute concentration until equilibrium is reached. B. In the cell, water passes in through aquaporins embedded in the cell membrane. Although water moves in both directions through aquaporins, there is a net movement of water from an area of lower to higher sugar concentration. Apply Concepts Does osmosis require the cell to use energy?
d▸ How Osmosis Works Look at the experimental setup in Figure 7–17A. The barrier is permeable to water but not to sugar. This means that water can cross the barrier in both directions, but sugar cannot. To start, there are more sugar molecules on the right side of the barrier than on the left side. Therefore, the concentration of water is lower on the right, where more of the solution is made of sugar. Although water molecules move in both directions across the membrane, there is a net movement of water toward the concentrated sugar solution.
Water will tend to move across the membrane until equilibrium is reached. At that point, the concentrations of water and sugar will be the same on both sides of the membrane. When this happens, the two solutions will be isotonic, which means “same strength.” Note that “strength” refers to the amount of solute, not water. When the experiment began, the more concentrated sugar solution (right side of the tube) was hypertonic, or “above strength,” compared to the left side. So the dilute sugar solution (left side of the tube) was hypotonic, or “below strength,” compared to the right side. Figure 7–17B shows how osmosis works across a cell membrane.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis