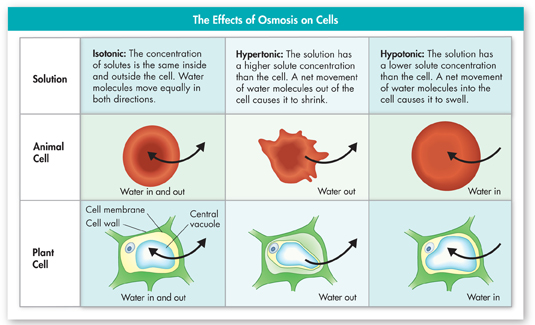
FIGURE 7–18 Osmotic Pressure Water molecules move equally into and out of cells placed in an isotonic solution. In a hypertonic solution, animal cells, like the red blood cell shown, shrink, and plant cell central vacuoles collapse. In a hypotonic solution, animal cells swell and burst. The central vacuoles of plant cells also swell, pushing the cell contents out against the cell wall. Predict What would happen to the cells of a saltwater plant if the plant were placed in fresh water?
d▸ Osmotic Pressure Driven by differences in solute concentration, the net movement of water out of or into a cell produces a force known as osmotic pressure. As shown in Figure 7–18, osmotic pressure can cause an animal cell in a hypertonic solution to shrink, and one in a hypotonic solution to swell. Because cells contain salts, sugars, proteins, and other dissolved molecules, they are almost always hypertonic to fresh water. As a result, water tends to move quickly into a cell surrounded by fresh water, causing it to swell. Eventually, the cell may burst like an overinflated balloon. In plant cells, osmotic pressure can cause changes in the size of the central vacuole, which shrinks or swells as water moves into or out of the cell.
Fortunately cells in large organisms are not in danger of bursting because most of them do not come in contact with fresh water. Instead, the cells are bathed in blood or other isotonic fluids. The concentrations of dissolved materials in these isotonic fluids are roughly equal to those in the cells themselves.
What happens when cells do come in contact with fresh water? Some, like the eggs laid in fresh water by fish and frogs, lack water channels. As a result, water moves into them so slowly that osmotic pressure is not a problem. Others, including bacteria and plant cells, are surrounded by tough walls. The cell walls prevent the cells from expanding, even under tremendous osmotic pressure. Notice how the plant cell in Figure 7–18 holds its shape in both hypertonic and hypotonic solutions while the animal red blood cell does not. However, increased osmotic pressure makes plant cells extremely vulnerable to cell wall injuries.
 In Your Notebook In your own words, explain why osmosis is really just a special case of facilitated diffusion.
In Your Notebook In your own words, explain why osmosis is really just a special case of facilitated diffusion.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis