Ionic Bonds An ionic bond is formed when one or more electrons are transferred from one atom to another. Recall that atoms are electrically neutral because they have equal numbers of protons and electrons. An atom that loses electrons becomes positively charged. An atom that gains electrons has a negative charge. These positively and negatively charged atoms are known as ions.
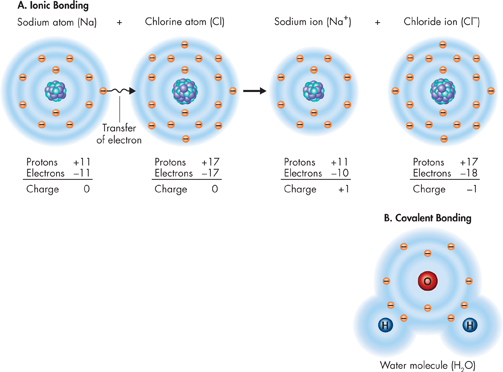
FIGURE 2–4 Ionic Bonding and Covalent Bonding A. The compound sodium chloride forms when sodium loses its valence electron to chlorine. B. In a water molecule, each hydrogen atom shares two electrons with the oxygen atom.
dFigure 2–4A shows how ionic bonds form between sodium and chlorine in table salt. A sodium atom easily loses its one valence electron and becomes a sodium ion (Na+). A chlorine atom easily gains an electron and becomes a chloride ion (Cl-). In a salt crystal, there are trillions of sodium and chloride ions. These oppositely charged ions have a strong attraction, forming an ionic bond.
Covalent Bonds Sometimes electrons are shared by atoms instead of being transferred. What does it mean to share electrons? It means that the moving electrons actually travel about the nuclei of both atoms, forming a covalent bond. When the atoms share two electrons, the bond is called a single covalent bond. Sometimes the atoms share four electrons and form a double bond. In a few cases, atoms can share six electrons, forming a triple bond. The structure that results when atoms are joined together by covalent bonds is called a molecule. The molecule is the smallest unit of most compounds. The diagram of a water molecule in Figure 2–4B shows that each hydrogen atom is joined to water's lone oxygen atom by a single covalent bond. When atoms of the same element join together, they also form a molecule. Oxygen molecules in the air you breathe consist of two oxygen atoms joined by covalent bonds.
 In Your Notebook In your own words, describe the differences between ionic and covalent bonds.
In Your Notebook In your own words, describe the differences between ionic and covalent bonds.

MYSTERY CLUE 
Fish do not break water molecules into their component atoms to obtain oxygen. Rather, they use oxygen gas dissolved in the water. How are the atoms in an oxygen molecule (O2) joined together?
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis