Van der Waals Forces Because of their structures, atoms of different elements do not all have the same ability to attract electrons. Some atoms have a stronger attraction for electrons than do other atoms. Therefore, when the atoms in a covalent bond share electrons, the sharing is not always equal. Even when the sharing is equal, the rapid movement of electrons can create regions on a molecule that have a tiny positive or negative charge.
When molecules are close together, a slight attraction can develop between the oppositely charged regions of nearby molecules. Chemists call such intermolecular forces of attraction van der Waals forces, after the scientist who discovered them. Although van der Waals forces are not as strong as ionic bonds or covalent bonds, they can hold molecules together, especially when the molecules are large.
ZOOMING IN
VAN DER WAALS FORCES AT WORK
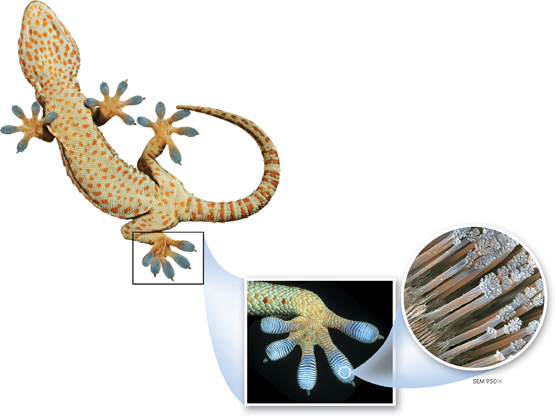
FIGURE 2–5 The underside of each foot on this Tokay gecko is covered by millions of tiny hairlike projections. The projections themselves are made of even finer fibers, creating more surface area for “sticking“ to surfaces at the molecular level. This allows geckos to scurry up walls and across ceilings.
2.1 Assessment

-
Review Describe the structure of an atom.
Infer An atom of calcium contains 20 protons. How many electrons does it have?
-
Review Why do all isotopes of an element have the same chemical properties?
Compare and Contrast Compare the structure of carbon–12 and carbon–14.
-
Review What is a compound?
Apply Concepts Water (H2O) and hydrogen peroxide (H2O2) both consists of hydrogen and oxygen atoms. Explain why they have different chemical and physical properties.
-
Review What are two types of bonds that hold the atoms within a compound together?
Classify A potassium atom easily loses its one valence electron. What type of bond will it form with a chlorine atom?
Apply the Big idea
Why do you think it is important that biologists have a good understanding of chemistry?

Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis