Energy in Reactions
 How do energy changes affect whether a chemical reaction will occur?
How do energy changes affect whether a chemical reaction will occur?
Energy is released or absorbed whenever chemical bonds are formed or broken. This means that chemical reactions also involve changes in energy.
Energy Changes Some chemical reactions release energy, and other reactions absorb it. Energy changes are one of the most important factors in determining whether a chemical reaction will occur.  Chemical reactions that release energy often occur on their own, or spontaneously. Chemical reactions that absorb energy will not occur without a source of energy. An example of an energy-releasing reaction is the burning of hydrogen gas, in which hydrogen reacts with oxygen to produce water vapor.
Chemical reactions that release energy often occur on their own, or spontaneously. Chemical reactions that absorb energy will not occur without a source of energy. An example of an energy-releasing reaction is the burning of hydrogen gas, in which hydrogen reacts with oxygen to produce water vapor.
The energy is released in the form of heat, and sometimes—when hydrogen gas explodes—light and sound.
The reverse reaction, in which water is changed into hydrogen and oxygen gas, absorbs so much energy that it generally doesn't occur by itself. In fact, the only practical way to reverse the reaction is to pass an electrical current through water to decompose water into hydrogen gas and oxygen gas. Thus, in one direction the reaction produces energy, and in the other direction the reaction requires energy.
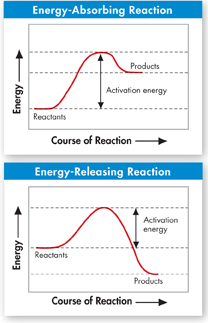
FIGURE 2–20 Activation Energy The peak of each graph represents the energy needed for the reaction to go forward. The difference between this required energy and the energy of the reactants is the activation energy. Interpret Graphs How do the energy of the reactants and products differ between an energy-absorbing reaction and an energy-releasing reaction?
Energy Sources In order to stay alive, organisms need to carry out reactions that require energy. Because matter and energy are conserved in chemical reactions, every organism must have a source of energy to carry out chemical reactions. Plants get that energy by trapping and storing the energy from sunlight in energy-rich compounds. Animals get their energy when they consume plants or other animals. Humans release the energy needed to grow tall, to breathe, to think, and even to dream through the chemical reactions that occur when we metabolize, or break down, digested food.
Activation Energy Chemical reactions that release energy do not always occur spontaneously. That's a good thing because if they did, the pages of this book might burst into flames. The cellulose in paper burns in the presence of oxygen and releases heat and light. However, paper burns only if you light it with a match, which supplies enough energy to get the reaction started. Chemists call the energy that is needed to get a reaction started the activation energy. As Figure 2–20 shows, activation energy is involved in chemical reactions regardless of whether the overall chemical reaction releases energy or absorbs energy.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis