Enzymes
 What role do enzymes play in living things and what affects their function?
What role do enzymes play in living things and what affects their function?
Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue. These chemical reactions are made possible by a process that would make any chemist proud—cells make catalysts. A catalyst is a substance that speeds up the rate of a chemical reaction. Catalysts work by lowering a reaction's activation energy.
Nature's Catalysts Enzymes are proteins that act as biological catalysts.  Enzymes speed up chemical reactions that take place in cells. Like other catalysts, enzymes act by lowering the activation energies, as illustrated by the graph in Figure 2–21. Lowering the activation energy has a dramatic effect on how quickly the reaction is completed. How big an effect does it have? Consider the reaction in which carbon dioxide combines with water to produce carbonic acid.
Enzymes speed up chemical reactions that take place in cells. Like other catalysts, enzymes act by lowering the activation energies, as illustrated by the graph in Figure 2–21. Lowering the activation energy has a dramatic effect on how quickly the reaction is completed. How big an effect does it have? Consider the reaction in which carbon dioxide combines with water to produce carbonic acid.
Left to itself, this reaction is so slow that carbon dioxide might build up in the body faster than the bloodstream could remove it. Your bloodstream contains an enzyme called carbonic anhydrase that speeds up the reaction by a factor of 10 million. With carbonic anhydrase on the job, the reaction takes place immediately and carbon dioxide is removed from the blood quickly.
Enzymes are very specific, generally catalyzing only one chemical reaction. For this reason, part of an enzyme's name is usually derived from the reaction it catalyzes. Carbonic anhydrase gets its name because it also catalyzes the reverse reaction that removes water from carbonic acid.
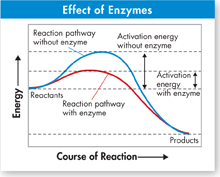
FIGURE 2–21 Effect of Enzymes Notice how the addition of an enzyme lowers the activation energy in this reaction. The enzyme speeds up the reaction.
d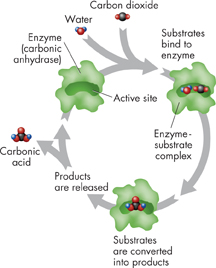
FIGURE 2–22 An Enzyme-Catalyzed Reaction The enzyme carbonic anhydrase converts the substrates carbon dioxide and water into carbonic acid (H2CO3). Predicting What happens to the carbonic anhydrase after the products are released?
ddThe Enzyme-Substrate Complex How do enzymes do their jobs? For a chemical reaction to take place, the reactants must collide with enough energy so that existing bonds will be broken and new bonds will be formed. If the reactants do not have enough energy, they will be unchanged after the collision.
Enzymes provide a site where reactants can be brought together to react. Such a site reduces the energy needed for reaction. The reactants of enzyme-catalyzed reactions are known as substrates. Figure 2–22 provides an example of an enzyme-catalyzed reaction.
Table of Contents
- Formulas and Equations
- Applying Formulas and Equations
- Mean, Median, and Mode
- Estimation
- Using Measurements in Calculations
- Effects of Measurement Errors
- Accuracy
- Precision
- Comparing Accuracy and Precision
- Significant Figures
- Calculating With Significant Figures
- Scientific Notation
- Calculating With Scientific Notation
- Dimensional Analysis
- Applying Dimensional Analysis