4.1 Studying Atoms
Reading Focus
Key Concepts
 What was Dalton's theory of the structure of matter?
What was Dalton's theory of the structure of matter? What contributions did Thomson and Rutherford make to the development of atomic theory?
What contributions did Thomson and Rutherford make to the development of atomic theory?
Vocabulary
nucleus
Reading Strategy
Summarizing Copy the table. As you read, complete the table about atomic models.
Scientist |
Evidence |
Model |
|---|---|---|
a. |
Ratio of masses in compounds |
b. |
c. |
Deflected beam |
d. |
Rutherford |
e. |
Positive, dense nucleus |
Studying the structure of atoms is a little like studying wind. Because you cannot see air, you must use indirect evidence to tell the direction of the wind. You might notice which way fallen leaves move as they are pushed by the wind, and infer that the leaves and wind are moving in the same direction.
Atoms pose a similar problem because they are extremely small. Even with a microscope, scientists cannot see the structure of an atom. In this chapter, you will find out how John Dalton, J. J. Thomson, Ernest Rutherford, Niels Bohr, and other scientists used evidence from experiments to develop models of atoms.
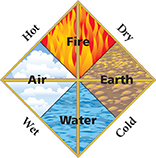
Figure 1 Aristotle thought that all substances were built up from only four elements—earth, air, fire, and water. These elements were a combination of four qualities—hot, cold, dry, and wet. Fire was a combination of hot and dry. Water was a combination of cold and wet.
 d
dAncient Greek Models of Atoms
If you cut a piece of aluminum foil in half, you have two smaller pieces of the same shiny, flexible substance. You could cut the pieces again and again. Can you keep dividing the aluminum into smaller pieces? Greek philosophers debated a similar question about 2500 years ago.
The philosopher Democritus believed that all matter consisted of extremely small particles that could not be divided. He called these particles atoms from the Greek word atomos, which means “uncut” or “indivisible.” He thought there were different types of atoms with specific sets of properties. The atoms in liquids, for example, were round and smooth, but the atoms in solids were rough and prickly.
Aristotle did not think there was a limit to the number of times matter could be divided. Figure 1 shows the model Aristotle used to describe matter. For many centuries, most people accepted Aristotle's views on the structure of matter. But by the 1800s, scientists had enough data from experiments to support an atomic model of matter.