CHAPTER 10 Assessment
Reviewing Content
Choose the letter that best answers the questions or completes the statement.
An alpha particle is identical to
a neutron.
a helium nucleus.
an electron.
a hydrogen nucleus.
When a beta particle is emitted, the mass number of a nucleus
increases by one.
decreases by one.
decreases by four.
remains the same.
The most penetrating form of nuclear radiation is
an alpha particle.
a beta particle.
a gamma ray.
an electron.
The half-life of cobalt-60 is 5.3 years. What fraction of a sample remains after 21.2 years?
one half
one quarter
one eighth
one sixteenth
Which of the following is a radioisotope commonly used in dating archeological artifacts?
nitrogen-14
carbon-12
uranium-235
carbon-14
Transmutation does not occur in which of these nuclear processes?
nuclear fission
nuclear fusion
alpha decay
gamma decay
Based on its location on the periodic table, an element that is not naturally occurring is
terbium (Tb).
curium (Cm).
holmium (Ho).
lutetium (Lu).
Nuclear particles are held together by
the strong nuclear force.
electrical attraction.
quarks.
electrical repulsion.
Nuclear power plants generate electricity from
nuclear fusion.
nuclear fission.
combustion.
radioactivity.
The primary reaction inside stars changes
hydrogen to helium.
helium to hydrogen.
uranium to plutonium.
nitrogen to carbon.
Understanding Concepts
How do radioisotopes of an element differ from other isotopes?
What is the effect on the mass number and charge of a nucleus when it loses an alpha particle?
How do the mass number and charge of a nucleus change when it emits a gamma ray?
Which type of radiation—alpha, beta, or gamma— is most dangerous to living things? Explain.
Why does a Geiger counter occasionally click even if no artificial radioisotopes are nearby?
How does raising the temperature affect the rate of nuclear decay?
Why can't carbon-14 be used to determine the age of fossils that are several hundred thousand years old?
Write the equation for the transmutation that occurs when an alpha particle combines with a nitrogen-14 atom, emitting a proton.
Compare and contrast the processes of fission and fusion.
What is necessary to sustain a nuclear chain reaction?
Why do nuclear reactions produce more energy per mass of matter than chemical reactions?
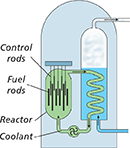
The diagram below shows a nuclear reactor, including control rods. What is the function of a control rod in a nuclear power plant?





 Interactive Textbook with assessment at
Interactive Textbook with assessment at