CHAPTER 3 Study Guide
3.1 Solids, Liquids, and Gases
 Key Concepts
Key Concepts
Materials can be classified as solids, liquids, or gases based on whether their shapes and volumes are definite or variable.
The kinetic theory of matter states that all particles of matter are in constant motion.
There are forces of attraction among the particles in all matter.
The constant motion of particles in a gas allows a gas to fill a container of any shape or size.
A liquid takes the shape of its container because particles in a liquid can flow to new locations. The volume of a liquid is constant because forces of attraction keep the particles close together.
Solids have a definite volume and shape because particles in a solid vibrate around fixed locations.
Vocabulary
solid, p. 69; liquid, p. 69; gas, p. 70; kinetic energy, p. 71
3.2 The Gas Laws
 Key Concepts
Key Concepts
Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas.
Factors that affect the pressure of an enclosed gas are its temperature, its volume, and the number of its particles.
Raising the temperature of a gas will increase its pressure if the volume of the gas and the number of particles are constant.
Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are constant.
Increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant.
The combined gas law can be expressed as
Vocabulary
pressure, p. 75; absolute zero, p. 78;
Charles's law, p. 78; Boyle's law, p. 79
 Key Concepts
Key Concepts
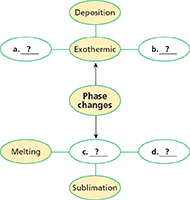
3.3 Phase Changes
Key Concepts
Melting, freezing, vaporization, condensation, sublimation, and deposition are six common phase changes.
The temperature of a substance does not change during a phase change.
Energy is either absorbed or released during a phase change.
The arrangement of molecules in water becomes less orderly as water melts, and more orderly as water freezes.
Evaporation takes place at the surface of a liquid and occurs at temperatures below the boiling point.
Vocabulary
phase change, p. 84; endothermic, p. 86;
heat of fusion, p. 86; exothermic, p. 86;
vaporization, p. 88; heat of vaporization, p. 88;
evaporation, p. 89; vapor pressure, p. 89;
condensation, p. 90; sublimation, p. 91;
deposition, p. 91;
Thinking Visually
Web Diagram Use information from the chapter to complete the web diagram on phase changes.