Explaining the Behavior of Gases
You can compare the motion of the particles in a gas to the movement of balls during a game of billiards. When a cue strikes a billiard ball, as shown in Figure 6, the ball moves in a straight line until it strikes the side of the billiard table or another ball. When a moving ball strikes a ball at rest, the first ball slows down and the second ball begins to move. Kinetic energy is transferred during those collisions.
Figure 6 This photograph of billiard balls was taken just after the cue struck the white ball, which began to move. The white ball moved in a straight line until it collided with the dark blue ball. The collision caused the dark blue ball to start moving. The motion of billiard balls can be compared to the motion of particles in a gas.

Motion in Gases
Unlike billiard balls, the particles in a gas are never at rest. At room temperature, the average speed of the particles in a sample of gas is about 1600 kilometers per hour. The use of the term average is a clue that not all particles are moving at the same speed. Some are moving faster than the average speed and some are moving slower than the average speed.
Figure 7 shows the possible paths of two helium atoms in a container of helium gas. Notice that each atom moves in a straight line until it collides with the other atom or with a wall of the container. During a collision, one atom may lose kinetic energy and slow down while the other atom gains kinetic energy and speeds up. However, the total kinetic energy of the atoms remains the same.
Figure 7 A helium atom travels in a straight line until it collides with another helium atom or the side of its container.
Relating Cause and Effect What can happen to the kinetic energy of two helium atoms when the atoms collide?
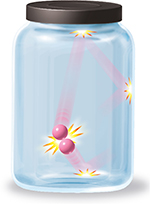
The diagram in Figure 7 does not accurately compare the volumes of the atoms and the container. The volume of a helium atom is extremely small compared to the volume of its container. If there were a billion times a trillion helium atoms in a liter bottle, there would still be a large amount of space in the bottle.
Between collisions, why doesn't one particle in a gas affect the other particles in the gas?  There are forces of attraction among the particles in all matter. If the particles are apart and moving fast, as in a gas, the attractions are too weak to have an effect. Under ordinary conditions, scientists can ignore the forces of attraction in a gas.
There are forces of attraction among the particles in all matter. If the particles are apart and moving fast, as in a gas, the attractions are too weak to have an effect. Under ordinary conditions, scientists can ignore the forces of attraction in a gas.




