Energy Levels
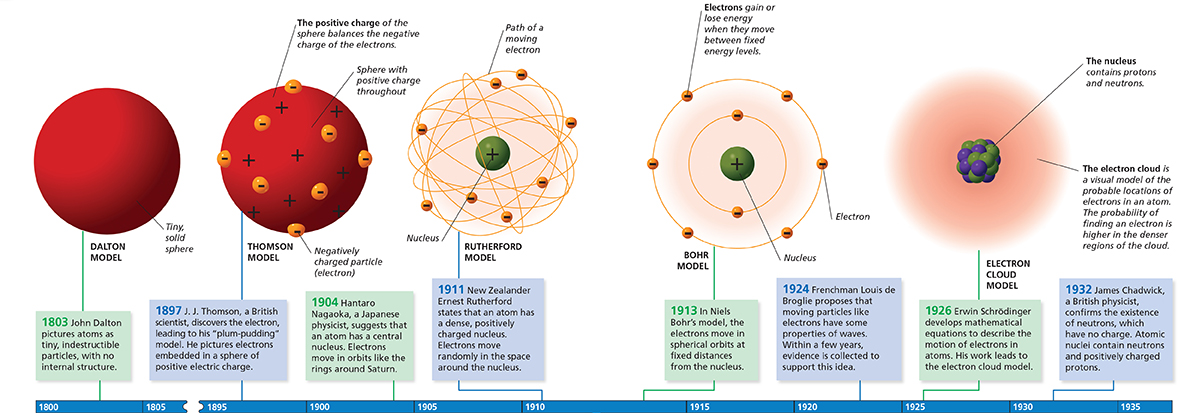
In Bohr's model, electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Each electron in an atom has a specific amount of energy. If an atom gains or loses energy, the energy of an electron can change. The possible energies that electrons in an atom can have are called energy levels.
To understand energy levels, picture them as steps in a staircase. As you move up or down the staircase, you can measure how your position changes by counting the number of steps you take. You might take one step up, or you might jump two steps down. Whether you are going up or down, you can move only in whole-step increments. Just as you cannot stand between steps on a staircase, an electron cannot exist between energy levels.
 SCIENCE and History
SCIENCE and History
Models of the Atom
The development of scientific ideas on the structure of atoms has passed several key milestones during the last 200 years.