The Effect of Size on Nuclear Forces
Electric forces in atomic nuclei depend on the number of protons. The greater the number of protons in a nucleus, the greater is the electric force that repels those protons. So in larger nuclei, the repulsive electric force is stronger than in smaller nuclei.
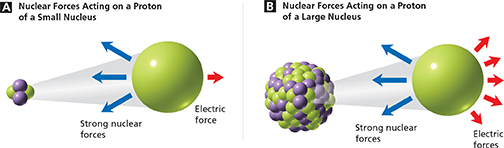
The effect of size on the strong nuclear force is more complicated. On one hand, the more protons and neutrons there are in a nucleus, the more possibilities there are for strong nuclear force attractions. However, as the size of the nucleus increases, the average distance between protons and neutrons increases. Because the strong nuclear force only acts over short ranges, the possibility of many attractions is never realized in a large nucleus. As a result, the strong nuclear force felt by one proton or neutron in a large nucleus is about the same as in a small nucleus, as shown in Figure 16.
Figure 16 The size of a nucleus affects how strongly it is bound together. A In a nucleus containing two protons and two neutrons, the strong nuclear forces easily overcome the electric force between the protons. B In a nucleus containing many protons and neutrons, the larger number of electric forces makes the nucleus less stable.
 d
dUnstable Nuclei
A nucleus becomes unstable, or radioactive, when the strong nuclear force can no longer overcome the repulsive electric forces among protons. While the strong nuclear force does not increase with the size of the nucleus, the electric forces do. There is, therefore, a point beyond which all elements are radioactive. All nuclei with more than 83 protons are radioactive.
Fission
In 1938, two German chemists, Otto Hahn and Fritz Strassman, performed a series of important transmutation experiments. By bombarding uranium-235 with high-energy neutrons, Hahn and Strassman hoped to produce more massive elements. Instead, their experiments produced isotopes of a smaller element, barium. Unable to explain their data, Hahn and Strassman turned to a colleague for help. In 1939, Lise Meitner, shown in Figure 17, and Otto Frisch, another physicist, offered a groundbreaking explanation for the experiments. The uranium-235 nuclei had been broken into smaller fragments. Hahn and Strassman had demonstrated nuclear fission. Fission is the splitting of an atomic nucleus into two smaller parts.
Figure 17 Austrian physicist Lise Meitner (1878—1968), shown here, and Otto Frisch were the first scientists to describe nuclear fission. Meitner correctly predicted that fission releases large amounts of energy.





