Quick Lab
Comparing Isomers

Materials
30 marshmallows, 70 raisins, 50 toothpicks
Procedure
Use marshmallows to represent carbon atoms, and raisins to represent hydrogen atoms, as in the model of propane shown. CAUTION Do not eat anything in the laboratory. Break the toothpicks in half to represent single bonds. Build models of five different isomers of hexane (C6H14).
For each model, attach all six carbon atoms first. Then attach hydrogen atoms until each carbon atom has four bonds.
Analyze and Conclude
Using Models Draw a structural formula for each isomer.
Predicting Would pentane (C5H12) or heptane (C7H16) have more isomers than hexane? Explain your answer.
Branched Chains
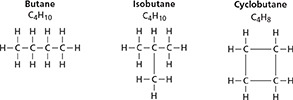
Look at the butane and isobutane formulas in Figure 5. Both compounds have a molecular formula of C4H10, but their structural formulas are different. In isobutane, there is a branch at the point where a carbon atom bonds to three other carbon atoms.
Compounds with the same molecular formula but different structural formulas are isomers. Differences in structure affect some properties of isomers. Butane boils at −0.5°C, but isobutane boils at −11.7°C. The number of possible isomers increases rapidly each time an additional carbon atom is added to the chain. For example, octane (C8H18) has 18 isomers, while decane (C10H22) has 75.
Rings
Figure 5 also shows the structural formula for cyclobutane. The carbon atoms in cyclobutane are linked in a four-carbon ring. Because each carbon atom forms bonds with two other carbon atoms, it can bond with only two hydrogen atoms. So cyclobutane (C4H8) molecules have two fewer hydrogen atoms than butane (C4H10) molecules. Most ring alkanes, or cyclic hydrocarbons, have rings with five or six carbons.
Figure 5 Butane, isobutane, and cyclobutane represent three ways that carbon atoms are arranged in an alkane—in a straight chain, in a branched chain, and in a ring. Classifying Explain why butane and cyclobutane are not isomers.
 d
d



