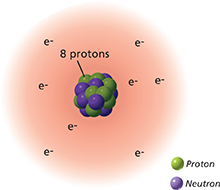
Figure 2 shows how charges are arranged in an atom. A cloud of negatively charged electrons surrounds the positively charged nucleus. The atom is neutral because it has an equal number of positive and negative charges. If an atom gains one or more electrons, it becomes a negatively charged ion. If an atom loses electrons, it becomes a positively charged ion.  An excess or shortage of electrons produces a net electric charge.
An excess or shortage of electrons produces a net electric charge.
Figure 2 A neutral atom has equal numbers of protons and electrons.
Drawing Conclusions What is the overall charge if the atom loses an electron?
 d
dThe SI unit of electric charge is the coulomb (C). It takes about electrons to produce a single coulomb. A lightning bolt is about 10 to 20 coulombs of charge. In comparison, a flash camera uses the energy from 0.025 coulombs of charge to produce each flash.
Electric Forces
Rub an inflated rubber balloon on your clean, dry hair. If it's a dry day, you can use the balloon to pick up bits of paper. The balloon attracts the paper because the balloon is negatively charged and the paper is positively charged. Now rub a second balloon on your hair and bring the two balloons close together. You can feel the balloons repel. The two balloons repel because they are negatively charged.  Like charges repel, and opposite charges attract. The force of attraction or repulsion between electrically charged objects is electric force.
Like charges repel, and opposite charges attract. The force of attraction or repulsion between electrically charged objects is electric force.
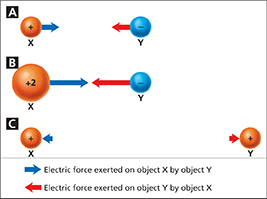
The French scientist Charles-Augustin de Coulomb (1736–1806) discovered that electric forces obey a law similar to the law of universal gravitation. The electric force between two objects is directly proportional to the net charge on each object and inversely proportional to the square of the distance between them. As you can see in Figure 3, doubling the net charge on one object doubles the electric force. If instead you double the distance between the objects, the electric force is one fourth as strong.
Inside an atom, electric forces are much stronger than gravitational forces. Electric forces form chemical bonds, which must be overcome in chemical changes. Electric forces also cause friction and other contact forces. But on a large scale, matter is mostly neutral and in that case, electric forces are close to zero.
Figure 3 Electric force depends on charge and distance. A Opposite charges attract each other. B Doubling one charge doubles the force on both charges. C Like charges repel. Doubling the distance makes the force one fourth as great.
 d
d What is electric force?
What is electric force?




