Figure 15 The brass vase on the left is coated with an oxide of copper that is red. Most of the surface of the plate on the right is coated with an oxide of copper that is black.
Classifying How can you be sure that the oxides of copper are different compounds?

Describing Ionic Compounds
Both of the objects in Figure 15 are coated with compounds of copper and oxygen. Based on the two colors of the coatings, copper and oxygen must form at least two compounds. One name cannot describe all the compounds of copper and oxygen. There must be at least two names to distinguish red copper oxide from black copper oxide.  The name of an ionic compound must distinguish the compound from other ionic compounds containing the same elements. The formula of an ionic compound describes the ratio of the ions in the compound.
The name of an ionic compound must distinguish the compound from other ionic compounds containing the same elements. The formula of an ionic compound describes the ratio of the ions in the compound.
Binary Ionic Compounds
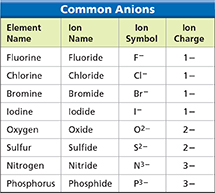
A compound made from only two elements is a binary compound. (The Latin prefix bi-means “two,” as in bicycle or bisect.) Naming binary ionic compounds, such as sodium chloride and cadmium iodide, is easy. The names have a predictable pattern: the name of the cation followed by the name of the anion. Remember that the name for the cation is the name of the metal without any change: sodium atom and sodium ion. The name for the anion uses part of the name of the nonmetal with the suffix –ide: iodine atom and iodide ion. Figure 16 shows the names and charges for eight common anions.
Figure 16 The table lists the element names, ion names, symbols, and charges for eight anions. The name of an anion is formed by adding the suffix –ide to the stem of the name of the nonmetal.
 dd
dd



