Critical Thinking
Comparing and Contrasting Compare what happens when NaCl and HCl are added to water.
Calculating The molar mass of sucrose, or table sugar, is 342 grams. Calculate how many grams of sucrose are required to make 3.0 liters of 0.50 M sucrose solution.
Applying Concepts A solution is an acid or a base, and it doesn't react with metal. Is its pH more likely to be 4 or 9? Explain your answer.
Inferring You have equal amounts of three colorless liquids, X, Y, and Z. An indicator is yellow in a pH of 8 or less and blue in a pH of 8 or more. The indicator turns blue in X and yellow in Y and Z. When you add liquid Z to X, the indicator turns yellow. When you add Z to Y, the solution remains yellow. Which liquid could be water?
Math Skills
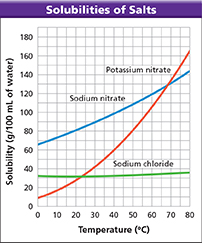
Use the graph to answer Questions 30−32.
Using Graphs Which compound is the most soluble at 75°C?
Interpreting Diagrams What kind of solution would you have if it contained 50 grams of sodium chloride in 100 mL of water at 30°C?
Calculating How many grams of sodium nitrate would you need to make 100 mL of a saturated solution at 25°C?
Concepts in Action
Designing an Experiment Commercials often say that antacids “neutralize excess stomach acid.” Stomach acid is hydrochloric acid with a pH of around 1− 4 depending on what and how recently you have eaten. Design an experiment to test how effective various brands of antacids are at neutralizing stomach acid.
Making Judgments A single dose of antacid A changes the pH of stomach contents from 1 to 3. A single dose of antacid B changes the pH of stomach contents from 1 to 8. Which antacid would you choose to use? Explain your answer.
Writing in Science You are in charge of writing the directions for a safety workers' training manual. The manual tells workers what to do if a train carrying nitric acid derails and the acid spills. What are some things they could do to clean up the spill? Write the procedures in steps, explaining why each step is done.
Performance-Based Assessment
Making a Display Prepare a classroom display on the importance of acids and bases in daily life. Include information on the properties of acids and bases, as well as examples of where they are found in daily life. Research ways in which manufacturers use acids and bases to produce common items and include that information in your display.