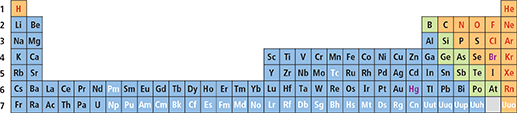
Figure 6 This diagram shows one way to display a periodic table of the elements. There are 7 rows, or periods, in the table. There are 32 columns, or groups, in the table. Comparing and Contrasting Compare the numbers of elements in Periods 1, 3, and 5.
The Periodic Law
Mendeleev developed his periodic table before the discovery of protons. He did not know that all atoms of an element have the same number of protons. He did not know that atoms of two different elements could not have the same number of protons.  In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Figure 6 shows one way the known elements can be arranged in order by increasing atomic number.
In the modern periodic table, elements are arranged by increasing atomic number (number of protons). Figure 6 shows one way the known elements can be arranged in order by increasing atomic number.
Periods
Each row in the table of elements in Figure 6 is a period. Period 1 has 2 elements. Periods 2 and 3 have 8 elements. Periods 4 and 5 have 18 elements. Period 6 has 32 elements. The number of elements per period varies because the number of available orbitals increases from energy level to energy level.
To understand the structure of the table, think about what happens as the atomic number increases. The first energy level has only one orbital. The one electron in a hydrogen atom and the two electrons in a helium atom can fit in this orbital. But one of the three electrons in a lithium atom must be in the second energy level. That is why lithium is the first element in Period 2. Sodium, the first element in Period 3, has one electron in its third energy level. Potassium, the first element in Period 4, has one electron in its fourth energy level. This pattern applies to all the elements in the first column on the table.
Groups
Each column on the periodic table is called a group. The elements within a group have similar properties.  Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. The elements in a group have similar electron configurations. An element's electron configuration determines its chemical properties. Therefore, members of a group in the periodic table have similar chemical properties. This pattern of repeating properties is the periodic law.
Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. The elements in a group have similar electron configurations. An element's electron configuration determines its chemical properties. Therefore, members of a group in the periodic table have similar chemical properties. This pattern of repeating properties is the periodic law.
Look at Figure 7 on pages 132 and 133. There are 18 groups in this periodic table. Some elements from Periods 6 and 7 have been placed below Period 7 so that the table is more compact.





