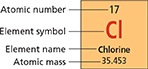
Figure 8 This box provides four pieces of information about the element chlorine: its symbol, its name, its atomic number, and its atomic mass.
Atomic Mass
There are four pieces of information for each element in Figure 7: the name of the element, its symbol, its atomic number and its atomic mass.  Atomic mass is a value that depends on the distribution of an element's isotopes in nature and the masses of those isotopes. You will use atomic masses when you study chemical reactions in Chapter 7.
Atomic mass is a value that depends on the distribution of an element's isotopes in nature and the masses of those isotopes. You will use atomic masses when you study chemical reactions in Chapter 7.
Atomic Mass Units
The mass of an atom in grams is extremely small and not very useful because the samples of matter that scientists work with contain trillions of atoms. In order to have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard. Recall that each isotope of an element has a different number of neutrons in the nuclei of its atoms. So the atoms of two isotopes have different masses.
Scientists assigned 12 atomic mass units to the carbon-12 atom, which has 6 protons and 6 neutrons. An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom.
Isotopes of Chlorine
In nature, most elements exist as a mixture of two or more isotopes. Figure 8 shows that the element chlorine has the symbol Cl, atomic number 17, and an atomic mass of 35.453 atomic mass units. (The unit for atomic mass is not listed in the periodic table, but it is understood to be the amu.) Where does the number 35.453 come from? There are two natural isotopes of chlorine, chlorine-35 and chlorine-37. An atom of chlorine-35 has 17 protons and 18 neutrons. An atom of chlorine-37 has 17 protons and 20 neutrons. So the mass of an atom of chlorine-37 is greater than the mass of an atom of chlorine-35.
Weighted Averages
Your teacher may use a weighted average to determine your grade. In a weighted average, some values are more important than other values. For example, test scores may count more heavily toward your final grade than grades on quizzes or grades on homework assignments.
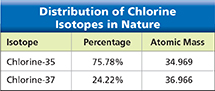
Figure 9 lists the atomic masses for two naturally occurring chlorine isotopes. If you add the atomic masses of the isotopes and divide by 2, you get 35.967, not 35.453. The value of the atomic mass for chlorine in the periodic table is a weighted average. The isotope that occurs in nature about 75% of the time (chlorine-35) contributes three times as much to the average as the isotope that occurs in nature about 25% of the time (chlorine-37).
Figure 9 This table shows the distribution and atomic masses for the two natural isotopes of chlorine. Using Tables Which isotope occurs more often in nature?
 d
d




 What is an atomic mass unit?
What is an atomic mass unit?