Melting and Freezing
In water, hydrogen and oxygen atoms are combined in small units called molecules. Each water molecule contains two hydrogen atoms and one oxygen atom.  The arrangement of molecules in water becomes less orderly as water melts and more orderly as water freezes.
The arrangement of molecules in water becomes less orderly as water melts and more orderly as water freezes.
Melting
In ice, attractions between water molecules keep the molecules in fixed positions. When ice cubes are removed from a freezer and placed in an empty glass, heat flows from the air to the ice. As the ice gains energy, the molecules vibrate more quickly. At the melting point of water, 0°C, some molecules gain enough energy to overcome the attractions and move from their fixed positions. When all the molecules have enough energy to move, melting is complete. Any energy gained by the water after the phase change increases the average kinetic energy of the molecules, and the temperature rises.
Freezing
When liquid water is placed in a freezer, energy flows from the water to the air in the freezer, and the water cools down. As the average kinetic energy of its molecules decreases, they move more slowly. At the freezing point of water, some molecules move slowly enough for the attractions between molecules to have an effect. When all the molecules have been drawn into an orderly arrangement, freezing is complete. Any energy removed from the ice after the phase change decreases the average kinetic energy of the molecules, and the temperature of the ice drops.
Often, people think of cold temperatures when they hear the term freezing. But substances that are solids at room temperature can freeze at temperatures that are quite high. For example, silicon freezes at 1412°C (2574°F). As a comparison, you can bake cookies at 177°C (350°F).
Vaporization and Condensation
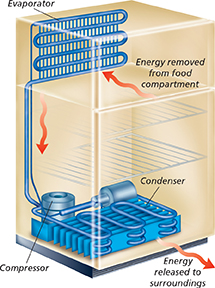
Figure 20 shows how food cools and stays cold in a refrigerator. The process depends on a substance that changes from a liquid to a gas to a liquid over and over again. During these phase changes, energy flows from the inside of the refrigerator to the outside.
The phase change in which a substance changes from a liquid into a gas is vaporization. Vaporization is an endothermic process. That is, a substance must absorb energy in order to change from a liquid to a gas. One gram of water gains 2258 joules of energy when it vaporizes at 100°C. This amount of energy is the heat of vaporization for water. The heat of vaporization varies from substance to substance.
Figure 20 In a refrigerator, a pair of phase changes keep the food cold. Energy from inside the food compartment is used to change a liquid to a gas in the evaporator. This energy is released when the compressed gas changes back to a liquid in the condenser.