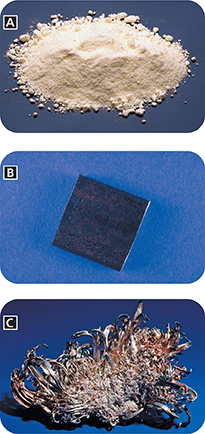
Figure 11 Each element has a different atomic number. A The atomic number of sulfur (S) is 16. B The atomic number of iron (Fe) is 26. C The atomic number of silver (Ag) is 47.
Applying Concepts How many protons are there in each atom of sulfur, iron, and silver?
Everything scientists know about the nucleus and subatomic particles is based on how the particles behave. Scientists still do not have an instrument that can show the inside of an atom. But they do have microscopes that can show how atoms are arranged on the surface of a material. The How It Works box on page 111 describes one of those microscopes.

Which scientist demonstrated the existence of neutrons?
Atomic Number and Mass Number
Dalton predicted that the atoms of any element are different from the atoms of all other elements. With the discovery of subatomic particles, scientists were able to describe those differences.
Atomic Number
The atoms of any given element always have the same number of protons. For example, there is one proton in the nucleus of each and every hydrogen atom. Therefore, hydrogen is assigned the atomic number 1. The atomic number of an element equals the number of protons in an atom of that element.
Hydrogen atoms are the only atoms with a single proton.  Atoms of different elements have different numbers of protons. The sulfur shown in Figure 11A is assigned atomic number 16 because a sulfur atom has 16 protons. You can use atomic numbers to refer to elements, like names and symbols, because each element has a unique atomic number.
Atoms of different elements have different numbers of protons. The sulfur shown in Figure 11A is assigned atomic number 16 because a sulfur atom has 16 protons. You can use atomic numbers to refer to elements, like names and symbols, because each element has a unique atomic number.
Each positive charge in an atom is balanced by a negative charge because atoms are neutral. So the atomic number of an element also equals the number of electrons in an atom. Each hydrogen atom has one electron. Each sulfur atom has 16.
Mass Number
The atomic number tells you the number of protons in an atom's nucleus. It does not give you any information about the number of neutrons in an atom. For that information, you need to know the atom's mass number. The mass number of an atom is the sum of the protons and neutrons in the nucleus of that atom. An atom of aluminum with 13 protons and 14 neutrons has a mass number of 27. If you know the atomic number and the mass number of an atom, you can find the number of neutrons by subtracting.
Number of Neutrons
Number of neutrons = Mass number − Atomic number




