SI Units of Measurement
For a measurement to make sense, it requires both a number and a unit. For example, if you told one of your friends that you had finished a homework assignment “in five,” what would your friend think? Would it be five minutes or five hours? Maybe it was a long assignment, and you actually meant five days. Or maybe you meant that you wrote five pages. You should always express measurements in numbers and units so that their meaning is clear. In Figure 12, students are measuring temperature in degrees Celsius.
Figure 12 A measurement consists of a number and a unit. One of the units used to measure temperature is the degree Celsius.

Many of the units you are familiar with, such as inches, feet, and degrees Fahrenheit, are not units that are used in science.  Scientists use a set of measuring units called SI, or the International System of Units. The abbreviation stands for the French name Système International d'Unités. SI is a revised version of the metric system, which was originally developed in France in 1791. By adhering to one system of units, scientists can readily interpret one another's measurements.
Scientists use a set of measuring units called SI, or the International System of Units. The abbreviation stands for the French name Système International d'Unités. SI is a revised version of the metric system, which was originally developed in France in 1791. By adhering to one system of units, scientists can readily interpret one another's measurements.
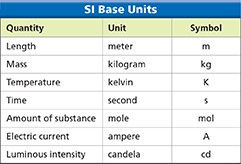
Base Units and Derived Units
SI is built upon seven metric units, known as base units, which are listed in Figure 13. In SI, the base unit for length, or the straight-line distance between two points, is the meter (m). The base unit for mass, or the quantity of matter in an object or sample, is the kilogram (kg).
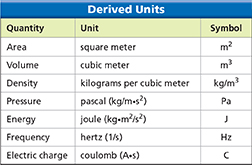
Additional SI units, called derived units, are made from combinations of base units. Figure 14 lists some common derived units. For example, volume is the amount of space taken up by an object. The volume of a rectangular box equals its length times its width times its height. Each of these dimensions can be measured in meters, so you can derive the SI unit for volume by multiplying meters by meters by meters, which gives you cubic meters (m3).