CHAPTER 6 Assessment
Reviewing Content
Choose the letter that best answers the question or completes the statement.
When an atom loses an electron, it forms a(n)
anion.
cation.
polyatomic ion.
neutral ion.
The charge on a chloride ion in AlCl3 is
1+.
3+.
1−.
3−.
Which pair has the same electron configuration?
Cl− and Ar
Cland Ar−
Cl and Ar
Cl+ and Ar
A chemical bond that forms when atoms share electrons is always a(n)
polar bond.
ionic bond.
metallic bond.
covalent bond.
When two fluorine atoms share a pair of electrons, the bond that forms is a(n)
polar covalent bond.
ionic bond.
nonpolar covalent bond.
double bond.
The chemical formula for magnesium bromide is
MgBr.
MgBr2.
Mg(II)Br2.
Mg2Br.
The compound with the formula SiCl4 is
silicon chloride.
silicon chlorine.
silicon(I) chloride.
silicon tetrachloride.
The attraction among water molecules is stronger than the attraction among
sodium and chloride ions.
carbon dioxide molecules.
the atoms in a polyatomic ion.
atoms in a diatomic molecule.
Which type of solid is likely to be the best conductor of electric current?
ionic compound
covalent compound
metal element
nonmetal element
An alloy contains
at least one metallic element.
at least one nonmetallic element.
only metallic elements.
only nonmetallic elements.
Understanding Concepts
What is a stable electron configuration?
What does each dot in an electron dot diagram represent?
What process changes atoms into ions?
What keeps the ions in their fixed positions within a crystal lattice?
What are subscripts used for in chemical formulas?
Explain why a melted ionic compound is a good conductor of electric current, but a solid ionic compound is a poor conductor of electric current.
What distinguishes single, double, and triple covalent bonds?
Explain why the covalent bonds in molecules of elements are always nonpolar.
Explain why, in a covalent bond between oxygen and hydrogen, the hydrogen atom has a partial positive charge and the oxygen atom has a partial negative charge.
What is the name of the binary compound formed from potassium and iodine?
Write the formulas for the compounds called copper(I) chloride and copper(II) chloride.
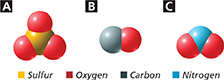
Name the compounds represented by the spacefilling models labeled A, B, and C.
In general, what determines the strength of metallic bonds?
What properties of copper and tin change when these metals are mixed together to form bronze?
What advantage of magnesium is retained in magnesium alloys? What disadvantage is reduced?





