Critical Thinking
Classifying What does a fluoride ion have in common with a neon atom and a sodium ion?
Comparing and Contrasting How are molecules and polyatomic ions similar?
Classifying Classify the bonds in each of these compounds as ionic, polar covalent, or nonpolar covalent: SO3, CaO, and I2.
Applying Concepts Write the names for the compounds with these chemical formulas: SCl2, Ag2SO4, LiF, CS2, and Ca(OH)2.
Use these diagrams to answer Questions 30–34.

Using Models Which of the three elements are metals and which are nonmetals?
Applying Concepts Element Q forms compounds with element X and with element Z. Write the formulas for these two compounds.
Calculating What would the formula be for a compound containing chromium(III) ions and ions of element Z?
Applying Concepts Draw an electron dot structure for a compound of fluorine and Z.
Predicting If an atom of X reacts with an atom of X, what kind of bond forms?
Math Skills
Calculating What is the total number of shared electrons in a carbon dioxide molecule?
Making Generalizations What is the ratio of anions to cations in a compound formed by a Group 2A metal and a Group 7A nonmetal?
Applying Concepts Write the formulas for barium fluoride, sodium oxide, iron(II) sulfate, and ammonium sulfate.
Concepts In Action
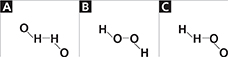
Using Models A solution of hydrogen peroxide (H2O2) and water is sometimes used to disinfect a cut. Which of the following formulas is the correct structural formula for hydrogen peroxide?
Relating Cause and Effect In a carbonated beverage, the main ingredients are water and carbon dioxide. Carbon dioxide gas is released when the bottle is opened. Why is water a liquid but carbon dioxide a gas at room temperature?
Classifying The shells shown on page 156 contain the compound calcium carbonate (CaCO3). Explain how this compound can contain both ionic and covalent bonds.
Relating Cause and Effect How does adding some phosphorus to silicon make silicon a better conductor of electric current?
Writing in Science Compare what happens to the valence electron in a hydrogen atom when the atom bonds with another hydrogen atom and when the atom bonds with an oxygen atom.
Performance-Based Assessment
Designing an Advertisement You own a store that sells bronze bells. Design a quarter-page ad for your store to be published in your local directory of businesses. Write copy for your ad. Describe a photograph to use in the ad. Also supply a sketch showing how you want the copy and the photograph to be laid out on the page.





