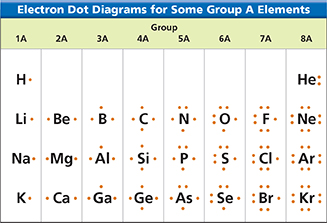
Figure 2 In an electron dot diagram, each dot represents a valence electron. Observing How many valence electrons do sodium and chlorine have?
 d
dIonic Bonds
Elements that do not have complete sets of valence electrons tend to react. By reacting, they achieve electron configurations similar to those of noble gases.  Some elements achieve stable electron configurations through the transfer of electrons between atoms.
Some elements achieve stable electron configurations through the transfer of electrons between atoms.
Transfer of Electrons
Look at the electron dot diagram for chlorine in Figure 2. A chlorine atom has one electron fewer than an argon atom. If the chlorine atom were to gain a valence electron, it would have the same stable electron arrangement as argon. Look at the electron dot diagram for sodium. A sodium atom has one more electron than a neon atom. If a sodium atom were to lose this electron, its highest occupied energy level would have eight electrons. It would then have the same stable electron arrangement as neon.
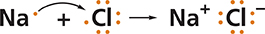
What happens at the atomic level when sodium reacts with chlorine? An electron is transferred from each sodium atom to a chlorine atom. Each atom ends up with a more stable electron arrangement than it had before the transfer.
Formation of Ions
When an atom gains or loses an electron, the number of protons is no longer equal to the number of electrons. The charge on the atom is not balanced and the atom is not neutral. An atom that has a net positive or negative electric charge is called an ion. The charge on an ion is represented by a plus or a minus sign. Notice the plus sign next to the symbol for sodium and the minus sign next to the symbol for chlorine.





