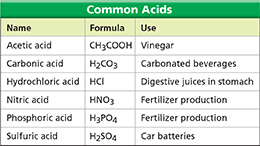
Figure 15 The table lists names, formulas, and uses for several common acids. Inferring What products are formed when nitric acid ionizes in water?
 d
dAcids have certain chemical and physical properties that are similar.  Some general properties of acids include sour taste, reactivity with metals, and ability to produce color changes in indicators.
Some general properties of acids include sour taste, reactivity with metals, and ability to produce color changes in indicators.
Sour Taste
Foods that taste sour often contain acids. For example, lemons, grapefruits, limes, and oranges all contain citric acid. The vinegar used in salad dressings contains acetic acid, CH3COOH. Dairy products that have spoiled contain butyric (byoo THIR ik) acid. While many of the foods you eat contain acids, you should never test an acid by tasting it.
Reactivity With Metals
When you use aluminum foil to cover a bowl of leftover spaghetti sauce or other foods containing tomatoes, the foil often turns dark. The foil may also develop small holes, and the food may acquire a metallic taste. Tomatoes contain citric acid, which reacts with metals such as aluminum.
The reaction between an acid and a metal is an example of a singlereplacement reaction. For example, when zinc is added to a test tube containing hydrochloric acid, bubbles form in the tube. The following equation describes the reaction.
As the zinc replaces hydrogen in hydrochloric acid, hydrogen gas and zinc(II) chloride are produced.
Color Changes in Indicators
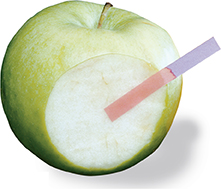
An indicator is any substance that changes color in the presence of an acid or base. One of the most common indicators used is litmus, a kind of dye derived from plants called lichens (LY kens). Litmus paper, shown in Figure 16, is made by coating strips of paper with litmus. Blue litmus paper turns red in the presence of an acid. If you drop an unknown solution onto blue litmus paper and the litmus paper turns red, you can classify the solution as an acid.
Figure 16 Litmus paper is an indicator that changes color in the presence of acids and bases. When blue litmus paper touches an acid, it turns red. Apples contain several acids, including malic acid, ascorbic acid (vitamin C), and citric acid.