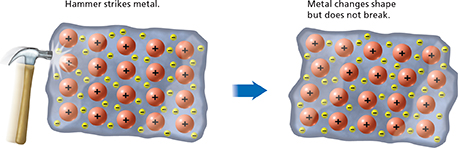
Figure 22 In a metal, cations are surrounded by shared valence electrons. If a metal is struck, the ions move to new positions, but the ions are still surrounded by electrons. Classifying What property of metals is displayed when a hammer strikes a metal?
 The cations in a metal form a lattice that is held in place by strong metallic bonds between the cations and the surrounding valence electrons. Although the electrons are moving among the atoms, the total number of electrons does not change. So, overall, the metal is neutral.
The cations in a metal form a lattice that is held in place by strong metallic bonds between the cations and the surrounding valence electrons. Although the electrons are moving among the atoms, the total number of electrons does not change. So, overall, the metal is neutral.
The metallic bonds in some metals are stronger than in other metals. The more valence electrons an atom can contribute to the shared pool, the stronger the metallic bonds will be. The bonds in an alkali metal are relatively weak because alkali metals contribute only a single valence electron. The result is that alkali metals, such as sodium, are soft enough to cut with a knife and have relatively low melting points. Sodium melts at 97.8°C. Transition metals, such as tungsten, have more valence electrons to contribute and, therefore, are harder and have higher melting points. Recall that tungsten melts at 3410°C.
Explaining Properties of Metals
The structure within a metal affects the properties of metals.  The mobility of electrons within a metal lattice explains some of the properties of metals. The ability to conduct an electric current and malleability are two important properties of metals.
The mobility of electrons within a metal lattice explains some of the properties of metals. The ability to conduct an electric current and malleability are two important properties of metals.
Recall that a flow of charged particles is an electric current. A metal has a built-in supply of charged particles that can flow from one location to another—the pool of shared electrons. An electric current can be carried through a metal by the free flow of the shared electrons.
The lattice in a metal is flexible compared to the rigid lattice in an ionic compound. Figure 22 is a model of what happens when someone strikes a metal with a hammer. The metal ions shift their positions and the shape of the metal changes. But the metal does not shatter because ions are still held together by the metallic bonds between the ions and the electrons. Metallic bonds also explain why metals, such as tungsten and copper, can be drawn into thin wires without breaking.

What two important properties of metals can be explained by their structure?




