Properties of Liquid Solutions
The physical properties of salt are clearly different from the physical properties of water. But how do the properties of a saltwater solution compare to those of its solute and solvent?  Three physical properties of a solution that can differ from those of its solute and solvent are conductivity, freezing point, and boiling point.
Three physical properties of a solution that can differ from those of its solute and solvent are conductivity, freezing point, and boiling point.
Conductivity
Solid sodium chloride is a poor conductor of electric current. But when sodium chloride dissociates in water, the sodium and chloride ions are able to move freely. The ions in solution will then conduct an electric current. Hydrogen chloride gas is also a poor conductor of electric current. However, when hydrogen chloride ionizes in water, the resulting solution conducts an electric current.
Freezing Point and Boiling Point
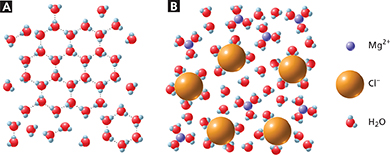
If you live in a cold climate, you are probably familiar with icy roads like the one in Figure 5. You may have seen snowplows or salt trucks spreading magnesium chloride, MgCl2, or a similar ionic compound on these icy roads. When magnesium chloride dissolves in melting ice and snow, it dissociates into magnesium (Mg2+) ions and chloride (Cl−) ions. As Figure 6A shows, ice forms when water molecules are able to arrange themselves in a rigid, honeycomb-like structure. In Figure 6B, the presence of magnesium and chloride ions, which are attracted to the water molecules, interferes with the freezing process. The freezing point of water at sea level is 0°C. When icy roads are salted with magnesium chloride, the resulting solution can have a freezing point as low as −15°C.
Figure 5 Salt spread on icy roads lowers the freezing point of water.

A solute can also raise the boiling point of the solvent. For example, the coolant used in most car radiators is a solution containing water and ethylene glycol, C2H6O2. Water at sea level boils at 100°C. Adding ethylene glycol to water raises the boiling point. The resulting solution helps prevent the engine from overheating. Because ethylene glycol also lowers the freezing point of water, the coolant does not freeze during spells of cold weather.
Figure 6 The presence of solute particles affects how a solvent freezes. A Pure water freezes in a hexagonal pattern. B In water “salted” with MgCl2, the dissociated Mg2+ and Cl− ions disrupt the formation of ice crystals. Using Models How do the interactions between Mg2+ and H2O differ from the interactions between Cl− and H2O?