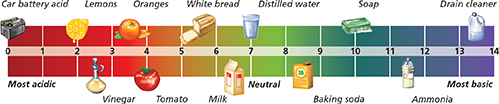
Figure 22 The pH scale can help you classify solutions as acids or bases.
Comparing and Contrasting The desired pH range of chlorinated water in swimming pools is 7.2 to 7.8. How does the concentration of hydronium ions in this solution compare to that of lemon juice?
 d
dThe pH Scale
Chemists use a number scale from 0 to 14 to describe the concentration of hydronium ions in a solution. It is known as the pH scale. The pH of a solution is a measure of its hydronium ion concentration. A pH of 7 indicates a neutral solution. Acids have a pH less than 7. Bases have a pH greater than 7.
Notice in Figure 22 that water falls in the middle of the pH scale. Water ionizes slightly according to the following reaction.
The arrow pointing to the left is longer than the arrow pointing to the right to show that water contains more molecules than ions. Water is neutral because it contains small but equal concentrations of hydronium ions and hydroxide ions. At 25°C, the concentration of both H3O+ and OH− in water is . Pure water has a pH of 7.
If you add an acid to water, the concentration of H3O+ increases and the concentration of OH− decreases. Suppose you have a hydrochloric acid solution in which the concentration of H3O+ is 0.10 M (). The solution has a pH of 1.  The lower the pH value, the greater the H3O+ ion concentrtion in solution is.
The lower the pH value, the greater the H3O+ ion concentrtion in solution is.
If you add a base to water, the concentration of OH− increases and the concentration of H3O+ decreases. Consider a sodium hydroxide solution in which the concentration of OH− is 0.10 M. The concentration of H3O+ in this solution is , which corresponds to a pH of 13.  The higher the pH value, the lower the H3O+ion concentration is.
The higher the pH value, the lower the H3O+ion concentration is.
 What is the pH of pure water?
What is the pH of pure water?
Strong Acids and Bases
Recall that some reactions go to completion while others reach equilibrium. When certain acids and bases dissolve in water, the formation of ions from the solute almost goes to completion. Such acids and bases are classified as strong.





