Math Skills
Balancing Chemical Equations
Write a balanced equation for the reaction between copper and oxygen to produce copper(II) oxide, CuO.
Read and Understand
What information are you given?
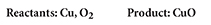
Plan and Solve
Write a chemical equation with the reactants on the left side and the product on the right.
This equation is not balanced. Change the coefficient of CuO in order to balance the number of oxygen atoms.
Change the coefficient of Cu in order to balance the number of copper atoms.
Look Back and Check
Is your answer reasonable?
The number of atoms on the left equals the number of atoms on the right.
Math Practice
Hydrogen chloride, or HCl, is an important industrial chemical. Write a balanced equation for the production of hydrogen chloride from hydrogen and chlorine.
Balance the following chemical equations.
Ethylene, C2H4, burns in the presence of oxygen to produce carbon dioxide and water vapor. Write a balanced equation for this reaction.
Counting With Moles
How many shoes do you own? Because shoes come in twos, you would most likely count them by the pair rather than individually. The counting units you use depend on what you are counting. For example, you might count eggs by the dozen or paper by the ream.
Chemists also need practical units for counting things. Although you can describe a reaction in terms of atoms and molecules, these units are too small to be practical.  Because chemical reactions often involve large numbers of small particles, chemists use a counting unit called the mole to measure amounts of a substance.
Because chemical reactions often involve large numbers of small particles, chemists use a counting unit called the mole to measure amounts of a substance.
A mole (mol) is an amount of a substance that contains approximately particles of that substance. This number is known as Avogadro's number. In chemistry, a mole of a substance generally contains atoms, molecules, or ions of that substance. For instance, a mole of iron is atoms of iron.
Figure 5 Shoes are often counted by the pair, eggs by the dozen, and paper by the ream (500 sheets). To count particles of a substance, chemists use the mole .





