Figure 3 Water is a compound made up of the elements hydrogen and oxygen.

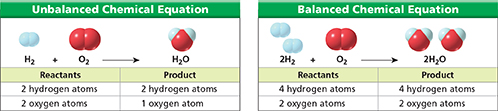
Figure 4 In the unbalanced equation, the hydrogen atoms are balanced but the oxygen atoms are not. After changing the coefficients, both the hydrogen and oxygen atoms are balanced.
Applying Concepts Why must chemical equations be balanced?
 d
dBalancing Equations
Water is formed by the reaction of hydrogen and oxygen. You can describe the reaction by writing a chemical equation.
If you examine this equation carefully, you will notice that the number of atoms on the left side does not equal the number of atoms on the right. The equation is not balanced.  In order to show that mass is conserved during a reaction, a chemical equation must be balanced.
In order to show that mass is conserved during a reaction, a chemical equation must be balanced.
You can balance a chemical equation by changing the coefficients, the numbers that appear before the formulas. In the unbalanced equation above, the coefficients are understood to be 1. When you change a coefficient, you change the amount of that reactant or product represented in the chemical equation. As you balance equations, you should never change the subscripts in a formula. Changing the formula changes the identity of that reactant or product.
The first step in balancing an equation is to count the number of atoms of each element on each side of the equation, as shown in Figure 4. The left side of the unbalanced equation has two hydrogen atoms and two oxygen atoms. The right side has two hydrogen atoms and one oxygen atom. The oxygen atoms need to be balanced.
The next step is to change one or more coefficients until the equation is balanced. You can balance the oxygen atoms by changing the coefficient of H2O to 2.
The oxygen atoms are now balanced. However, the hydrogen atoms have become “unbalanced,” with two on the left, and four on the right. To balance the hydrogen atoms, change the coefficient of H2 to 2.
The equation is now balanced. Each side of the balanced equation has four hydrogen atoms and two oxygen atoms, as shown in Figure 4. According to the balanced equation, two molecules of hydrogen react with one molecule of oxygen to yield two molecules of water.





