Wave or Particle?
Scientists know that electromagnetic radiation travels as a wave. Scientists also have evidence that electromagnetic radiation behaves like a stream of particles. In the late 1600s, the English physicist Isaac Newton was the first to propose a particle explanation. He based this hypothesis on two pieces of evidence: light travels in a straight line and it casts a shadow, as shown in Figure 4. But not all evidence supports Newton's hypothesis. So which is light, wave or particle? It is both.  Electromagnetic radiation behaves sometimes like a wave and sometimes like a stream of particles.
Electromagnetic radiation behaves sometimes like a wave and sometimes like a stream of particles.
Figure 4 The fact that light casts a shadow has been used as evidence for both the wave model of light and the particle model of light.

Evidence for the Wave Model
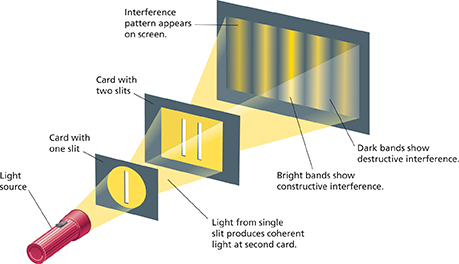
In 1801, the English physicist Thomas Young (1773—1829) showed that light behaves like a wave. Look at Figure 5. Young passed a beam of light first through a single slit and then through a double slit. Where light from the two slits reached a darkened screen, Young observed alternating bright and dark bands. The bands were evidence that the light had produced an interference pattern. Bright bands indicated constructive interference, and dark bands indicated destructive interference. Interference occurs only when two or more waves overlap. Therefore, Young's experiment showed that light behaves like a wave.
What is the evidence that light travels like a wave?
Figure 5 This diagram illustrates Young's experiment, which showed that light behaves like a wave. When light passes through a single slit and then a double slit, it produces an interference pattern. Constructive interference produces bright bands of light. Destructive interference produces dark bands.
Predicting What would you expect to see on the screen if light behaved like a stream of particles?
 d
d




