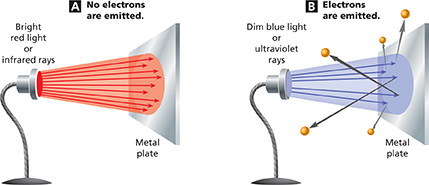
Figure 6 The emissions of electrons from a metal caused by light striking the metal is called the photoelectric effect. A Red light or infrared rays, no matter how bright, does not cause electrons to be emitted from this metal surface. B When blue light or ultraviolet rays strike the metal surface, electrons are emitted, even if the light is dim.
Evidence for the Particle Model
When dim blue light hits the surface of a metal such as cesium, an electron is emitted. A brighter blue light causes even more electrons to be emitted, as you can see in Figure 6. But red light, no matter how bright it is, does not cause the emission of any electrons in this particular metal.
The emission of electrons from a metal caused by light striking the metal is called the photoelectric effect. Discovered in 1887, the photoelectric effect was puzzling. Scientists did not understand why dim blue light caused electrons to be emitted from metal but even bright red light did not.
In 1905, Albert Einstein (1879—1955) proposed that light, and all electromagnetic radiation, consists of packets of energy. These packets of electromagnetic energy are now called photons (FOH tawnz). Each photon's energy is proportional to the frequency of the light. The greater the frequency of an electromagnetic wave, the more energy each of its photons has.
Blue light has a higher frequency than red light, so photons of blue light have more energy than photons of red light. Blue light consists of photons that have enough energy to cause electrons to be emitted from a metal surface. So blue light can cause emission of electrons.
Red light has a lower frequency than blue light, so photons of red light have less energy than photons of blue light. Red light consists of photons that have too little energy to cause any electrons to be emitted from a metal surface. So red light does not cause emission of electrons.
What is the photoelectric effect?





