Thermodynamics
The study of conversions between thermal energy and other forms of energy is called thermodynamics. Count Rumford made a good start in this field. But many scientists still believed that heat was a kind of matter. Then in 1845, James Prescott Joule (1818–1889) published his results from a convincing experiment.
Joule carefully measured the energy changes in a system. Recall that a system is any group of objects that interact with one another. Joule's system included a falling weight that turned a paddle wheel in a container of water. As the weight fell, the paddle churned a known mass of water. The water heated up due to friction from the turning paddle. Joule carefully measured the work done by the falling weight. He found that the work almost exactly equaled the thermal energy gained by the water. Joule is often given credit for discovering the first law of thermodynamics. That is the law of conservation of energy applied to work, heat, and thermal energy.
First Law of Thermodynamics
Recall that energy cannot be created or destroyed. But energy can be converted into different forms.  The first law of thermodynamics states that energy is conserved. If energy is added to a system, it can either increase the thermal energy of the system or do work on the system. But no matter what happens, all of the energy added to the system can be accounted for. Energy is conserved.
The first law of thermodynamics states that energy is conserved. If energy is added to a system, it can either increase the thermal energy of the system or do work on the system. But no matter what happens, all of the energy added to the system can be accounted for. Energy is conserved.
Look at the bicycle pump in Figure 9. You can consider the tire, the pump, and the air inside to be a system. The force exerted on the pump does work on the system. Some of this work is useful; it compresses air into the tire. The rest of the work is converted into thermal energy. That is why a bicycle pump heats up as you inflate a tire.
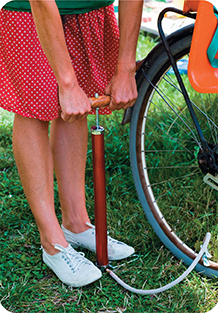
Figure 9 You can consider the bicycle pump, the tire, and the air inside of both to be a system. The person does work on the system by pushing on the pump. Some of the work is converted into thermal energy, which heats the air in the pump and the tire.
Second Law of Thermodynamics
If you take a cold drink from the refrigerator and leave it out in a warm room, will the drink become colder? Of course it won't. You know that the drink will warm up. Thermal energy flows spontaneously only from hotter to colder objects.
 The second law of thermodynamics states that thermal energy can flow from colder objects to hotter objects only if work is done on the system. A refrigerator, for example, must do work to transfer thermal energy from the cold food compartment to the warm room air. The thermal energy is released by coils at the bottom or in the back of the refrigerator.
The second law of thermodynamics states that thermal energy can flow from colder objects to hotter objects only if work is done on the system. A refrigerator, for example, must do work to transfer thermal energy from the cold food compartment to the warm room air. The thermal energy is released by coils at the bottom or in the back of the refrigerator.





