Proton Donors and Acceptors
Recall that hydronium ions (H3O+) are produced when acids dissolve in water. When an acid and a base react in water, a proton from the hydronium ion from the acid combines with the hydroxide ion (OH−) from the base to form water (H2O). Acids lose, or “donate,” protons. Bases “accept” protons, forming water, a neutral molecule.  Acids can be defined as proton donors, and bases can be defined as proton acceptors. This definition allows you to classify a wider range of substances as acids or bases.
Acids can be defined as proton donors, and bases can be defined as proton acceptors. This definition allows you to classify a wider range of substances as acids or bases.
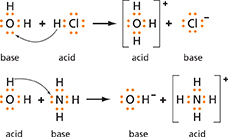
Figure 20 In the first reaction, water acts as a base, accepting a proton from hydrogen chloride. In the second reaction, water acts as an acid, donating a proton to the ammonia.
Applying Concepts What acts as the proton donor in the first reaction?
 dd
ddBased on the definitions of acids and bases that you read earlier in this section, water is neither an acid nor a base. However, using the proton-donor or proton-acceptor definition, water can act as either an acid or a base depending on the compound with which it reacts.
Figure 20 shows the ionization of hydrogen chloride and ammonia as they form solutions. In the first reaction, water acts as a base. It accepts a proton from hydrogen chloride and becomes a hydronium ion. In the second reaction, water acts as an acid. It donates a proton to the ammonia, which acts as a base. The resulting solution contains hydroxide ions and ammonium ions, NH4+.
Section 8.3 Assessment
Reviewing Concepts
 List three general properties of acids.
List three general properties of acids. List three general properties of bases.
List three general properties of bases. What are the two products of a neutralization reaction?
What are the two products of a neutralization reaction? What are the proton-donor and proton-acceptor definitions of acids and bases?
What are the proton-donor and proton-acceptor definitions of acids and bases? What ion is present in all common acid solutions?
What ion is present in all common acid solutions?
Critical Thinking
Using Analogies Commercials for antacids often claim these products neutralize stomach acid. Antacids are bases. Think of an analogy for the way in which antacids neutralize acids.
Applying Concepts In the following equation, which reactant is a proton donor? Which is a proton acceptor?
Connecting Concepts
Classifying Reactions Compare neutralization with the types of chemical reactions described in Section 7.2. To which type of reaction is neutralization most similar? Explain your choice.




