Radioisotopes of uranium—primarily uranium-238—were the source of radioactivity in Becquerel's experiment. (Recall that the name of an isotope includes its mass number.) Another common radioisotope is carbon-14, which can be found in fossils like the ones shown in Figure 2.
Unlike stable isotopes such as carbon-12 or oxygen-16, radioisotopes spontaneously change into other isotopes over time. When the composition of a radioisotope changes, the radioisotope is said to undergo nuclear decay.  During nuclear decay, atoms of one element can change into atoms of a different element altogether. For example, uranium-238 decays into thorium-234, which is also a radioisotope.
During nuclear decay, atoms of one element can change into atoms of a different element altogether. For example, uranium-238 decays into thorium-234, which is also a radioisotope.
Figure 2 About 26,000 years ago, more than 100 mammoths died at a sinkhole in Hot Springs, South Dakota. Scientists figured out how old the remains were by measuring amounts of the radioisotope carbon-14 contained in the mammoth bones.

Types of Nuclear Radiation
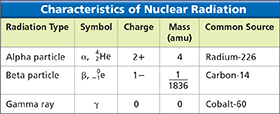
Scientists can detect a radioactive substance by measuring the nuclear radiation it gives off. Nuclear radiation is charged particles and energy that are emitted from the nuclei of radioisotopes.  Common types of nuclear radiation include alpha particles, beta particles, and gamma rays. Figure 3 shows the properties of these three types of radiation.
Common types of nuclear radiation include alpha particles, beta particles, and gamma rays. Figure 3 shows the properties of these three types of radiation.
Alpha Decay
When a uranium-238 sample decays, it emits alpha particles. An alpha particle is a positively charged particle made up of two protons and two neutrons—the same as a helium nucleus. It has a 2+ charge. The common symbol for an alpha particle is . The subscript is the atomic number (the number of protons). The superscript is the mass number (the sum of the numbers of protons and neutrons). Another symbol for an alpha particle is the Greek letter α.
Alpha decay, which refers to nuclear decay that releases alpha particles, is an example of a nuclear reaction. Like chemical reactions, nuclear reactions can be expressed as equations. The following nuclear equation describes the alpha decay of uranium-238.
In alpha decay, the product isotope has two fewer protons and two fewer neutrons than the reactant isotope. In the equation above, the mass number on the left (238) equals the sum of the mass numbers on the right . Also, the atomic number on the left (92) equals the sum of the atomic numbers on the right . In other words, the equation is balanced.
Alpha particles are the least penetrating type of nuclear radiation. Most alpha particles travel no more than a few centimeters in air, and can be stopped by a sheet of paper or by clothing.
Figure 3 Within a few years of Becquerel's discovery of radioactivity, Ernest Rutherford classified three types of nuclear radiation based on his own studies of uranium compounds. Comparing and Contrasting How do alpha particles, beta particles, and gamma rays differ in terms of charge? In terms of mass?
 ddd
ddd