Thermal Contraction and Expansion
If you take a balloon outside on a cold winter day, it shrinks. Can you explain why? As temperature decreases, the particles that make up the air inside the balloon move more slowly, on average. Slower particles collide less often and exert less force, so gas pressure decreases and the balloon contracts. This is called thermal contraction.
If you bring the balloon inside, it expands. Thermal expansion is an increase in the volume of a material due to a temperature increase.
 Thermal expansion occurs when particles of matter move farther apart as temperature increases. Gases expand more than liquids and liquids usually expand more than solids. A gas expands more easily than a liquid or a solid because the forces of attraction among particles in a gas are weaker.
Thermal expansion occurs when particles of matter move farther apart as temperature increases. Gases expand more than liquids and liquids usually expand more than solids. A gas expands more easily than a liquid or a solid because the forces of attraction among particles in a gas are weaker.
Thermal expansion is used in glass thermometers. As temperature increases, the alcohol in the tube expands and its height increases. The increase in height is proportional to the increase in temperature. In an oven thermometer, a strip of brass and a strip of steel are bonded together and wound up in a coil. As the coil heats up, the two metals expand at different rates, and the coil unwinds. This causes the needle to rotate on the temperature scale.
Specific Heat
When a car is heated by the sun, the temperature of the metal door increases more than the temperature of the plastic bumper. Do you know why? One reason is that the iron in the door has a lower specific heat than the plastic in the bumper. Specific heat is the amount of heat needed to raise the temperature of one gram of a material by one degree Celsius. If equal masses of iron and plastic absorb the same heat, the iron's temperature rises more.  The lower a material's specific heat, the more its temperature rises when a given amount of energy is absorbed by a given mass.
The lower a material's specific heat, the more its temperature rises when a given amount of energy is absorbed by a given mass.
Specific heat is often measured in joules per gram per degree Celsius, or J/g·°C. Figure 3 gives specific heats for a few common materials. It takes 4.18 joules of energy to raise the temperature of 1.00 gram of water by 1.00 degree Celsius. How much energy is needed to heat 2.00 grams of water to the same temperature? You would have to add twice as much energy, or 8.36 joules.
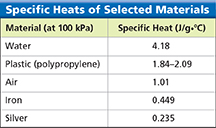
Figure 3 Specific heat is the heat needed to raise the temperature of 1 gram of material by 1°C. Analyzing Data Which material in the table has the highest specific heat? The lowest?
dMaterial (at 100 kPa) |
Specific Heat (J/gooC) |
Water |
4.18 |
Plastic (polypropylene) |
1.84–2.09 |
Air |
1.01 |
Iron |
0.449 |
Silver |
0.235 |






