6.4 The Structure of Metals
Reading Focus
Key Concepts
 What are the forces that give a metal its structure as a solid?
What are the forces that give a metal its structure as a solid? How do metallic bonds produce some of the typical properties of metals?
How do metallic bonds produce some of the typical properties of metals? How are the properties of alloys controlled?
How are the properties of alloys controlled?
Vocabulary
metallic bond
alloy
Reading Strategy
Relating Cause and Effect Copy the concept map. As you read, complete the map to relate the structure of metals to their properties.
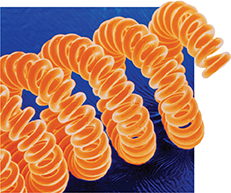
Figure 21 This photograph of the tungsten filament from a light bulb was taken with a scanning electron microscope. Color was added to the photo. The filament is magnified more than 100 times. The diameter of the wire is about 15 μm, or 0.0015 cm.
Light bulbs are easy to ignore unless a bulb burns out and you are searching for a replacement in the dark. But in the decades just before the year 1900, light bulbs were an exciting new technology. One challenge for researchers was to find the best material for the filaments in light bulbs. The substance had to be ductile enough to be drawn into a narrow wire. It could not melt at the temperatures produced when an electric current passes through a narrow wire. It had to have a low vapor pressure so that particles on the surface were not easily removed by sublimation.
The substance the researchers found was tungsten (W), a metal whose name means “heavy stone” in Swedish. Figure 21 shows a magnified view of the narrow coils in a tungsten filament. Tungsten has the highest melting point of any metal—3410°C—and it has the lowest vapor pressure. The properties of a metal are related to bonds within the metal.
Metallic Bonds
Metal atoms achieve stable electron configurations by losing electrons. But what happens if there are no nonmetal atoms available to accept the electrons? There is a way for metal atoms to lose and gain electrons at the same time. In a metal, valence electrons are free to move among the atoms. In effect, the metal atoms become cations surrounded by a pool of shared electrons. A metallic bond is the attraction between a metal cation and the shared electrons that surround it.