Attraction Between Molecules
In a molecular compound, there are forces of attraction between molecules. These attractions are not as strong as ionic or covalent bonds, but they are strong enough to hold molecules together in a liquid or solid.  Attractions between polar molecules are stronger than attractions between nonpolar molecules.
Attractions between polar molecules are stronger than attractions between nonpolar molecules.
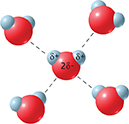
Water molecules are similar in mass to methane (CH4) molecules. Yet, methane boils at -161.5°C and water boils at 100°C because methane molecules are nonpolar and water molecules are polar. Each dashed line in Figure 13 represents an attraction between a partially positive hydrogen atom in one water molecule and a partially negative oxygen atom in another. Molecules on the surface of a water sample are attracted to molecules that lie below the surface and are pulled toward the center of the sample. These attractions increase the energy required for water molecules to evaporate. They raise the temperature at which vapor pressure equals atmospheric pressure—the boiling point.
Attractions among nonpolar molecules are weaker than attractions among polar molecules, but they do exist. After all, carbon dioxide can exist as solid dry ice. Attractions among nonpolar molecules explain why nitrogen can be stored as a liquid at low temperatures and high pressures. Because electrons are constantly in motion, there are times when one part of a nitrogen molecule has a small positive charge and one part has a small negative charge. At those times, one nitrogen molecule can be weakly attracted to another nitrogen molecule.
Figure 13 Each dashed line in the drawing represents an attraction between a hydrogen atom and an oxygen atom.
Interpreting Diagrams In a water molecule, which atom has a partial negative charge? Which has a partial positive charge?
 dd
ddSection 6.2 Assessment
Reviewing Concepts
 What attractions hold atoms together in a covalent bond?
What attractions hold atoms together in a covalent bond? What happens to the charge on atoms when they form a polar covalent bond?
What happens to the charge on atoms when they form a polar covalent bond? Name the two factors that determine whether a molecule is polar.
Name the two factors that determine whether a molecule is polar. Compare the strength of attractions between polar molecules to the strength of attractions between nonpolar molecules.
Compare the strength of attractions between polar molecules to the strength of attractions between nonpolar molecules.What is a molecule?
Critical Thinking
Applying Concepts Which of these elements does not bond to form molecules: oxygen, chlorine, neon, or sulfur?
Inferring Why is the boiling point of water higher than the boiling point of chlorine?
Using Diagrams Based on their electron dot diagrams, what is the formula for the covalently bonded compound of nitrogen and hydrogen?
Connecting Concepts
Viscosity Review the description of the physical property viscosity in Section 2.2. Then write a paragraph explaining how attractions between molecules might affect the viscosity of a liquid.




