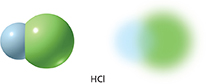
Figure 11 Shared electrons in a hydrogen chloride molecule spend less time near the hydrogen atom than near the chlorine atom. Inferring Which element has a greater attraction for electrons—hydrogen or chlorine?
Polar Covalent Bonds
In a molecule of an element, the atoms that form covalent bonds have the same ability to attract an electron. Shared electrons are attracted equally to the nuclei of both atoms. In a molecule of a compound, electrons may not be shared equally.
Figure 11 shows models of the molecule that forms when hydrogen reacts with chlorine. A chlorine atom has a greater attraction for electrons than a hydrogen atom does. In a hydrogen chloride molecule, the shared electrons spend more time near the chlorine atom than near the hydrogen atom. A covalent bond in which electrons are not shared equally is called a polar covalent bond. (One meaning of the term polar is “opposite in character, nature, or direction.”)
 When atoms form a polar covalent bond, the atom with the greater attraction for electrons has a partial negative charge. The other atom has a partial positive charge. The symbols δ and δ + are used to show which atom has which charge. (δ is the lowercase version of the Greek letter delta.)
When atoms form a polar covalent bond, the atom with the greater attraction for electrons has a partial negative charge. The other atom has a partial positive charge. The symbols δ and δ + are used to show which atom has which charge. (δ is the lowercase version of the Greek letter delta.)
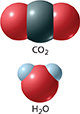
Figure 12 In a carbon dioxide (CO2) molecule, the polar bonds between the carbon atom and the oxygen atoms cancel out because the molecule is linear. In a water (H2O) molecule, the polar bonds between the oxygen atom and the hydrogen atoms do not cancel out because the molecule is bent.
Polar and Nonpolar Molecules
Can you assume that a molecule that contains a polar covalent bond is polar? If a molecule has only two atoms, it will be polar. But, when molecules have more than two atoms, the answer is not as obvious.  The type of atoms in a molecule and its shape are factors that determine whether a molecule is polar or nonpolar.
The type of atoms in a molecule and its shape are factors that determine whether a molecule is polar or nonpolar.
Compare the models of carbon dioxide and water in Figure 12. In carbon dioxide, there are double bonds between each oxygen atom and the central carbon atom. Because oxygen has a greater attraction for electrons than carbon does, each double bond is polar. However, the molecule is linear: all three atoms are lined up in a row. The carbonoxygen double bonds are directly opposite each other. There is an equal pull on the electrons from opposite directions. The pulls cancel out and the molecule as a whole is nonpolar.
There are two single bonds in a water molecule. The bonds are polar because oxygen has a greater attraction for electrons than hydrogen does. Because the water molecule has a bent shape rather than a linear shape, the polar bonds do not cancel out. The two hydrogen atoms are located on the same side of the molecule, opposite the oxygen atom. The oxygen side of the molecule has a partial negative charge. The hydrogen side of the molecule has a partial positive charge.




